Some (local) Historical Background.
 One starting point for the story is with 28 year-old Hermann Sachse.
One starting point for the story is with 28 year-old Hermann Sachse. 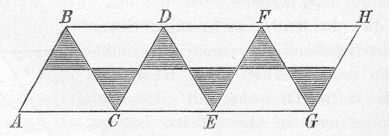 Following the recognition of the tetrahedral nature of saturated carbon in 1874 (or perhaps 1869),
Sachse in 1890 using only trigonometry,
derived the non-planar chair and boat conformations of cyclohexane and the axial and equatorial positions of the hydrogens.
He even encouraged readers of his articles to construct a paper model to help them visualise this! Conformational analysis was well and truly born
(except that no-one recognised the significance of Sachse's work, and he died in obscurity in 1893!). His work was eventually vindicated in 1918
when Ernst Mohr analysed the structure of
diamond, and showed that the carbon framework adopted Sachse's chair conformation.
Following the recognition of the tetrahedral nature of saturated carbon in 1874 (or perhaps 1869),
Sachse in 1890 using only trigonometry,
derived the non-planar chair and boat conformations of cyclohexane and the axial and equatorial positions of the hydrogens.
He even encouraged readers of his articles to construct a paper model to help them visualise this! Conformational analysis was well and truly born
(except that no-one recognised the significance of Sachse's work, and he died in obscurity in 1893!). His work was eventually vindicated in 1918
when Ernst Mohr analysed the structure of
diamond, and showed that the carbon framework adopted Sachse's chair conformation.
In 1950 (following a 1934 theoretical analysis by William Penney,
a future Rector of Imperial College), Derek Barton made conformational (and 3D) analysis a mainstay of organic chemistry,
applying it to great effect to understanding the chemistry of steroids, and eventually sharing the
Nobel prize in 1969 for this idea. This is commemorated in one of the graphical images
adorning the student services centre in the chemistry building at Imperial.
During the years ~1965-1975 he delivered the predecessor to this very course in person at Imperial College. Since then, the theoretical understanding of conformational analysis has continued to develop. Nowadays, the subject overlaps considerably with the theory and practice of molecular modelling and of stereoelectronics.
Two of the most famous Nobel-prize winning examples of science in the 20th century use Barton's principles of conformational analysis:
 The model of DNA built by Watson and Crick.
The model of DNA built by Watson and Crick. ,
, Linus Pauling's equally famous description of the structure of proteins.
Linus Pauling's equally famous description of the structure of proteins.
Nowadays, much of what is now known as molecular biology is founded on these two pillars. In this course, our 3D models are obtained from either the CCDC crystal database or by calculation.
© Henry S. Rzepa, 2010-2014. Hide|show Toolbar.
 One starting point for the story is with 28 year-old Hermann Sachse.
One starting point for the story is with 28 year-old Hermann Sachse.  Following the recognition of the tetrahedral nature of saturated carbon in 1874 (or perhaps 1869),
Sachse in 1890 using only trigonometry,
derived the non-planar chair and boat conformations of cyclohexane and the axial and equatorial positions of the hydrogens.
He even encouraged readers of his articles to construct a paper model to help them visualise this! Conformational analysis was well and truly born
(except that no-one recognised the significance of Sachse's work, and he died in obscurity in 1893!). His work was eventually vindicated in 1918
when Ernst Mohr analysed the structure of
diamond, and showed that the carbon framework adopted Sachse's chair conformation.
Following the recognition of the tetrahedral nature of saturated carbon in 1874 (or perhaps 1869),
Sachse in 1890 using only trigonometry,
derived the non-planar chair and boat conformations of cyclohexane and the axial and equatorial positions of the hydrogens.
He even encouraged readers of his articles to construct a paper model to help them visualise this! Conformational analysis was well and truly born
(except that no-one recognised the significance of Sachse's work, and he died in obscurity in 1893!). His work was eventually vindicated in 1918
when Ernst Mohr analysed the structure of
diamond, and showed that the carbon framework adopted Sachse's chair conformation.