 The app is thus stabilized by ~3.0 kcal/mol less than the gauche.
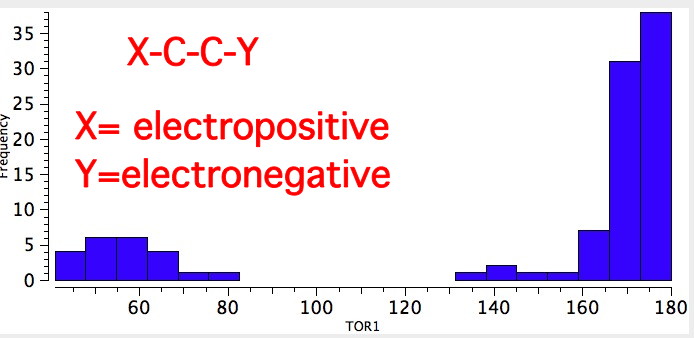
The app is thus stabilized by ~3.0 kcal/mol less than the gauche.In compounds of the generic structure, X-C-C-Y, where X and Y are both electronegative groups, the balance of conformational preference shifts from app to gauche
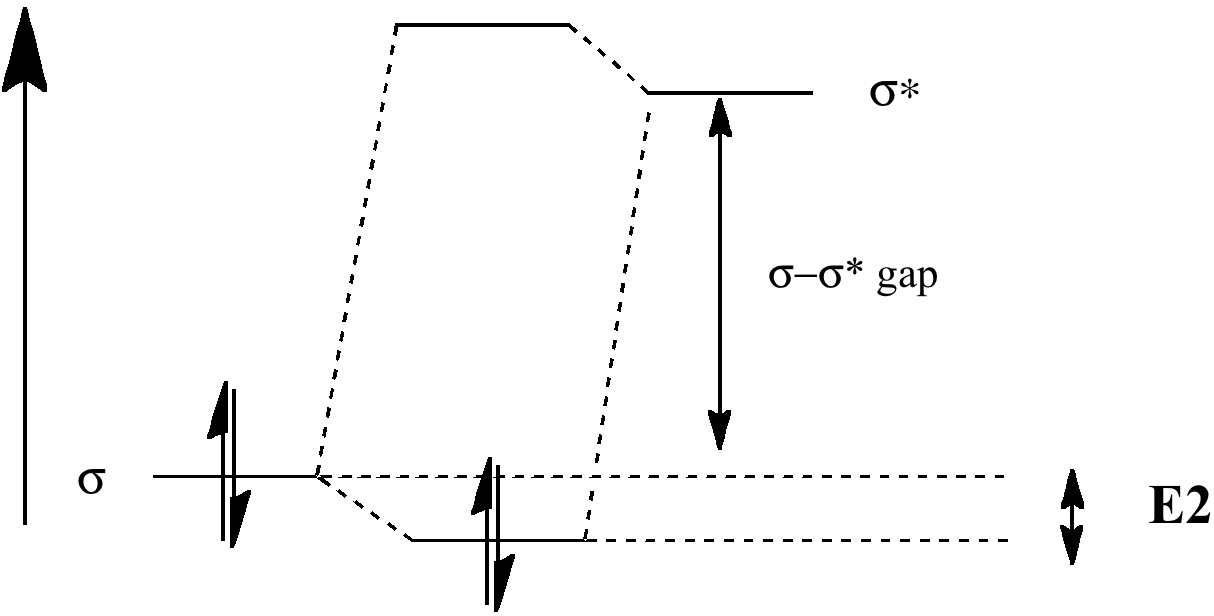
This is now due to the orbital overlap (effect 1 in theory) starting to dominate over the other two controlling factors. The relative energies of the σ-orbitals and σ*-orbitals of C-X or C-Y bonds have the following order:
| ⇐ small σ-σ* gap | large σ-σ* gap ⇒ | ||
|---|---|---|
| ⇐ Large E(2) | Small E(2) ⇒ | ||
| low energy acceptor: | σ*C-F < σ*C-Cl < σ*C-O ≈ σ*C-+NMe3 < σ*C-C≡N < σ*C-N < σ*C-C < σ*C-H < σ*C-Si | high energy acceptor: |
| high energy donor: | σC-Sn > σC-Si > σC-H > σC-C > σC-N > σC-O > σC-Cl > σC-F | low energy donor |
Thus vertical E(2) values on the left hand side of this list, for which the σ-σ* energy gap is small, are large whilst those on the rhs are much weaker:
| gauche | anti-periplanar (app) |
|---|---|
|
|
 The app is thus stabilized by ~3.0 kcal/mol less than the gauche.
The app is thus stabilized by ~3.0 kcal/mol less than the gauche.
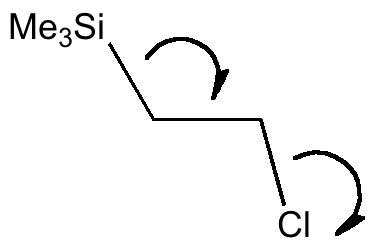
An example involving C-Si as a better electron donor than C-H is shown below. This time the effect is to favour the anti-periplanar conformation, since the Si-C bond is a good donor, and C-Cl* is a good acceptor empty orbital (but not the other way around, σC-Cl/σ*C-Si, E(2) = 2.3 kcal/mol)
| app conformation | gauche conformation |
|---|---|
| σC-Si/σ*C-Cl, E(2) = 9.2 σC-H/σ*C-H, E(2) = 3.8 |
σC-H/σ*C-Cl, E(2) = 8.8 kcal/mol, σC-Si/σ*C-H, E(2) = 3.3 kcal/mol |
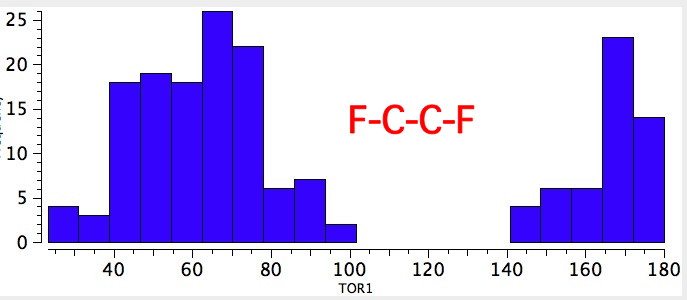
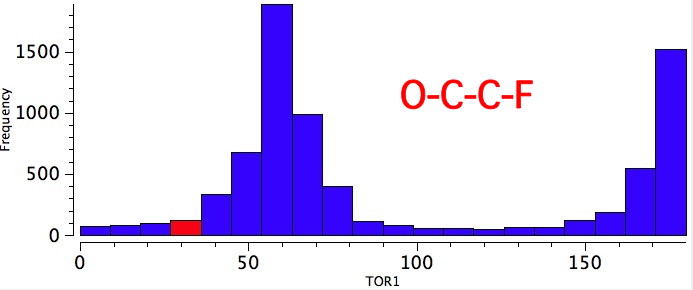
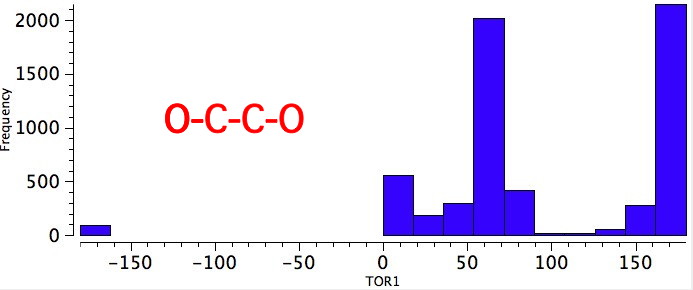
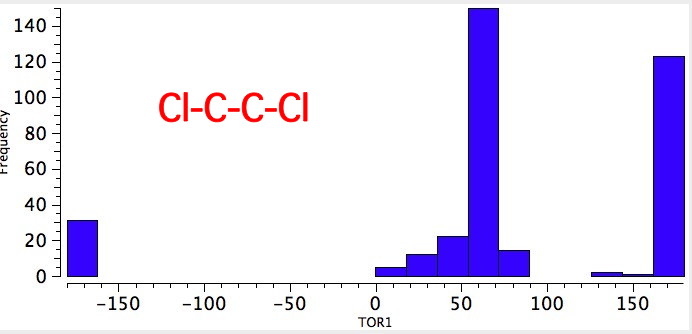
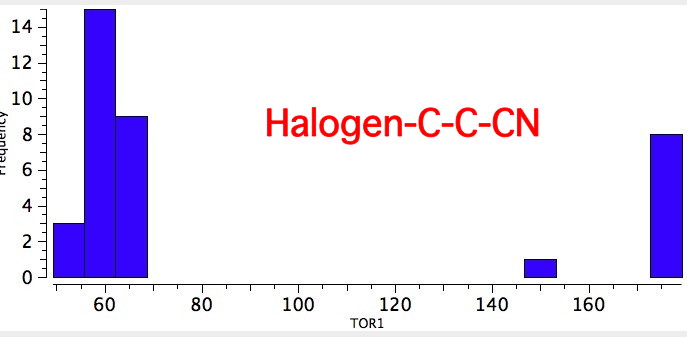
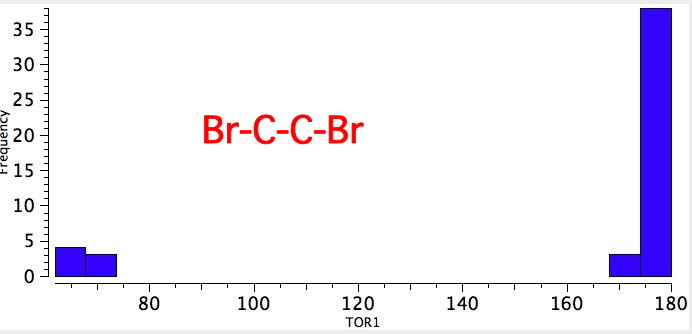
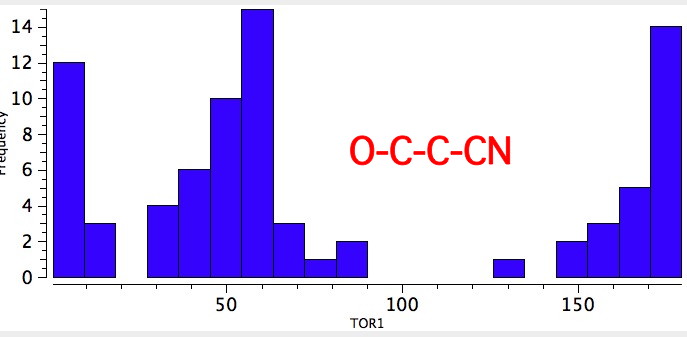
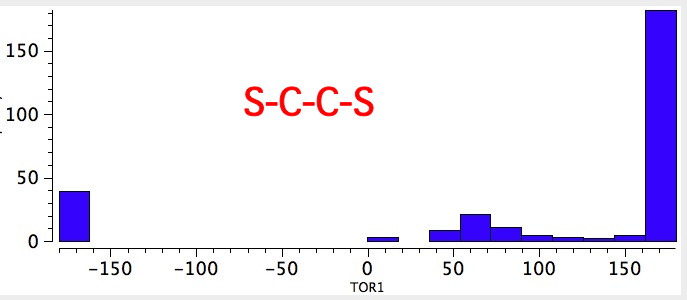
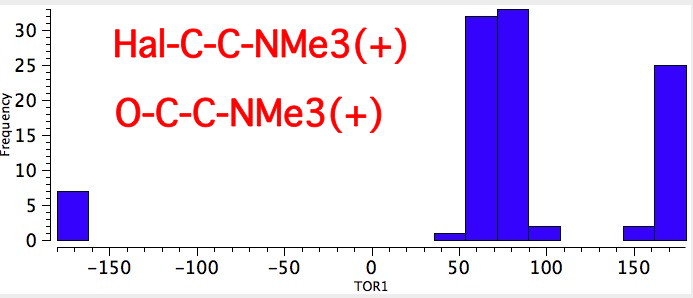
 As one moves down the periodic table, e.g. Sn-C is a better donor than Si-C. Conversely, Br-C is a much worse acceptor than F-C, and S-C is a worse acceptor than O-C. This effect also manifests in a search of the Cambridge crystal database for many combinations.
As one moves down the periodic table, e.g. Sn-C is a better donor than Si-C. Conversely, Br-C is a much worse acceptor than F-C, and S-C is a worse acceptor than O-C. This effect also manifests in a search of the Cambridge crystal database for many combinations.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
In the case of 1,2-ethane-diol an intramolecular H-bond may also (weakly) stabilize the gauche form. A strong hydrogen bond does not form, because the O-H...O angle cannot adopt the preferred value of ~180°. Hydrogen bonds can also be thought of as arising from an interaction between a donor (Lp) and an acceptor H-X σ* interaction, and the overlap of that interaction is optimal for a co-linear angle. In that sense, the gauche effect and linear hydrogen bonds arise from exactly the same type of orbital interaction. 
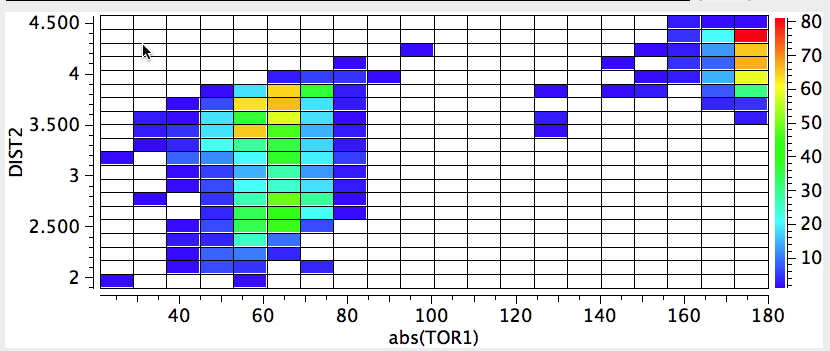
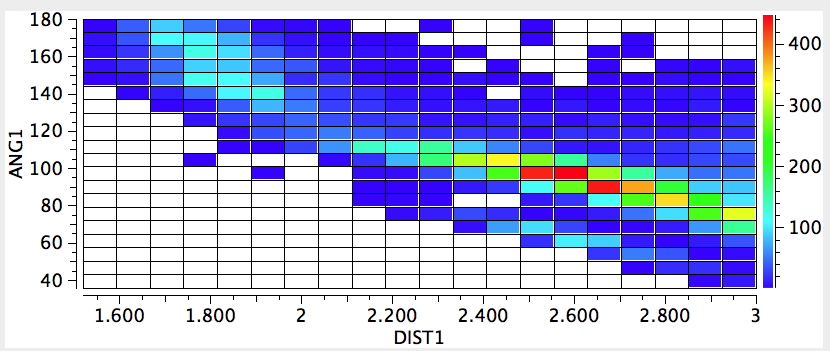
The record holder for the gauche effect is the molecule below:
 The rotational barrier for this molecule is > 25 kcal/mol, which gives it a reasonable kinetic life at
room temperatures (t½ ~ 100 hours@298K). It thus crosses the boundary between a conformational isomer and a configurational isomer. Yes, it is also chiral (perhaps the smallest molecule capable of being so) and potentially resolvable into enantiomers.
The rotational barrier for this molecule is > 25 kcal/mol, which gives it a reasonable kinetic life at
room temperatures (t½ ~ 100 hours@298K). It thus crosses the boundary between a conformational isomer and a configurational isomer. Yes, it is also chiral (perhaps the smallest molecule capable of being so) and potentially resolvable into enantiomers.
© Henry S. Rzepa, 2010-2014. Hide|show Toolbar.