Let us consider first in more detail molecule with which you should be familiar in conformational terms, namely, butane. The chart below summarises a number of terms in common usage in conformational studies, many of which you should have seen in the first year:
If the molecule is rotated in small increments (5°) about the central bond, the energy (for those who care, using a DFT method known as ωB97XD/6-311G(d,p)) shows up as follows.
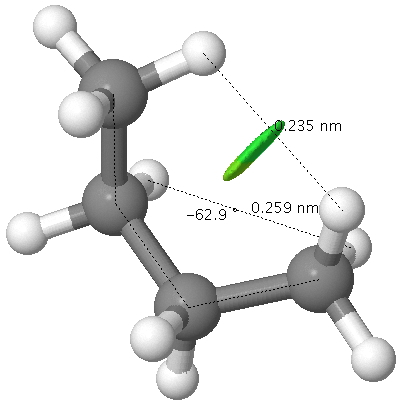
There are two limiting conformations of butane which have a minimum of energy on a plot of energy versus dihedral angle:

and two maxima in that plot which we call transition states connecting the conformations
| eclipsed/syn-periplanar transition state | eclipsed/anti-clinal transition state |
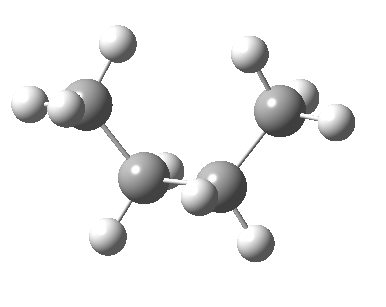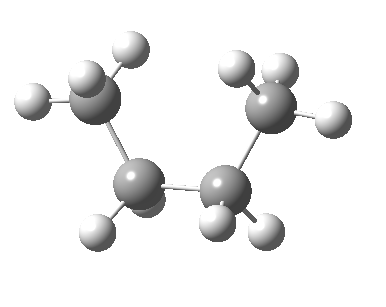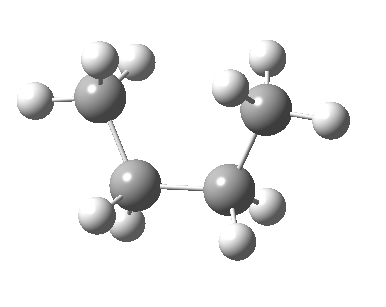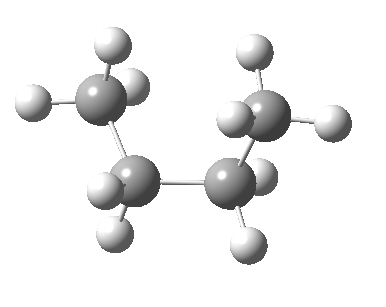 |
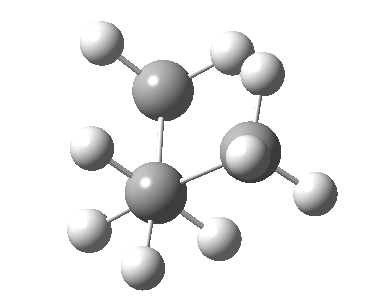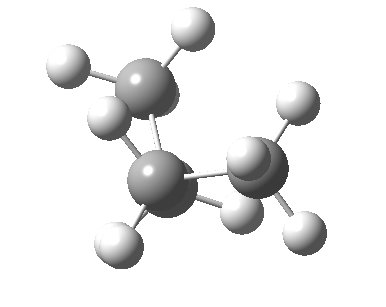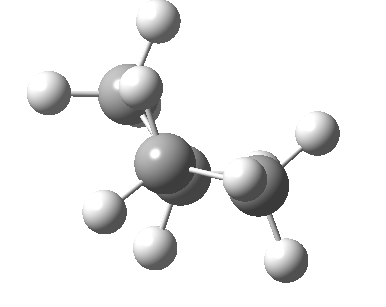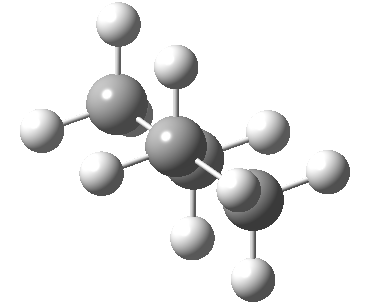 |
The higher energy of the eclipsed forms compared to the app or anti-periplanar form (3-5 kcal/mol) is defined as the strain and since it derives from twisting (or torsion) about single bonds this strain is called torsional strain. We shall see later that certain molecules cannot avoid a high degree of torsional strain, but for most straight chain molecules this strain can be relieved by rotation about the bond to give one of the staggered conformations.
Overall, the staggered/anti-periplanar conformation is ~0.47 kcal/mol more stable in free energy ΔG298 than the gauche form [ΔG = -RT Ln (69/31)]. Can we at least qualitatively explain this in terms of the three theoretical effects listed in the previous theoretical section?
Effects two and three ~cancel, which means the anti-periplanar conformation wins out through effect one (Note: ΔΔG298 is only very ~ ≡ ΔE(2)).
The rate of interconversion of the conformers is given by Ln(k/T) = 23.76 - ΔG/RT. For the app/gauche barrier at 298K (~ 3 kcal/mol), k = ~1010 s-1, which is FAST. Even at 100K, it is ~105 s-1. which is a half-life of ~ 0.000006 seconds. These very short half-lives are what characterise conformations (as opposed to configurations which have very long half-lives).
Given the relatively high proportion (31%) of the gauche) conformation in butane, we may expect similar conformations to contribute substantially in higher straight-chain molecules. Bartell and Kohl in 1963 (DOI: 10.1063/1.1734149) showed by electron diffraction of heptane in the gas phase that frequent gauche twists develop in the chain and they were able to estimate the relative proportions of the various conformations as shown in the following table.
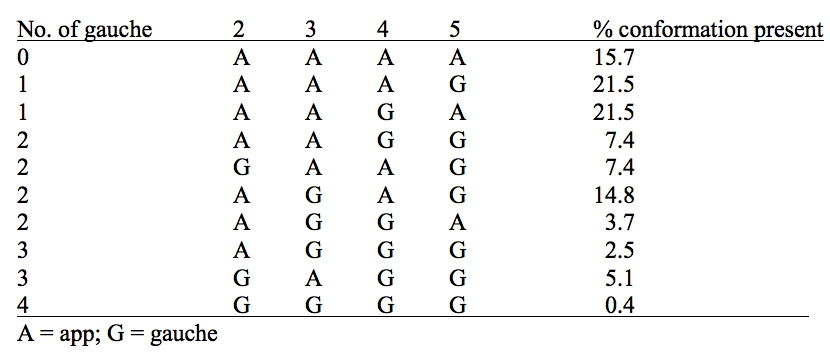
This incursion of gauche forms is due to the longer distances favouring folding of the chain back upon itself, and hence setting up van der Waals attractions. There are two particularly attractive H...H non-convalent interactions (NCI) resulting from the AGAG conformation, the dispersion energy of which is now 0.7 kcal/mol lower than for the AAAA conformation.
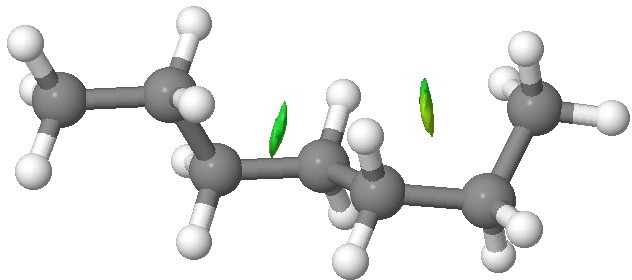
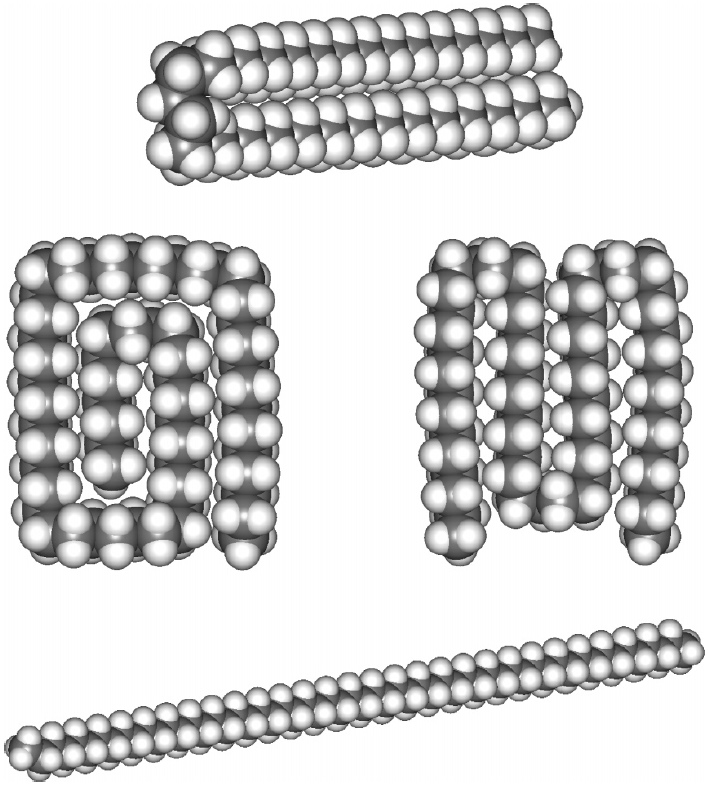
 Medium length alkanes have significant populations with one or more gauche conformations (DOI: 10.1021/jp064811m), and by ~C17, the hairpin gauche conformation has become the lowest energy and dominant form.
Medium length alkanes have significant populations with one or more gauche conformations (DOI: 10.1021/jp064811m), and by ~C17, the hairpin gauche conformation has become the lowest energy and dominant form.
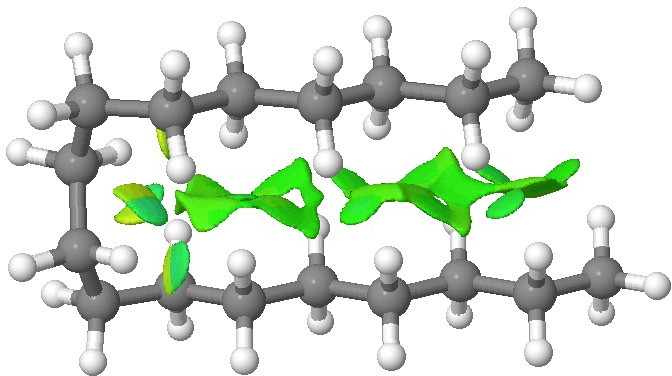
~C30 exhibits a double hairpin, and ~C34 a broken paperclip form. By C58, the hairpin shape is now as much as ~12 kcal/mol lower than the all-trans linear shape.
© Henry S. Rzepa, 2010-2014. Hide|show Toolbar.