Metathesis reactions are a series of catalysed transformations which transpose the atoms in alkenes or alkynes. Alkyne metathesis is closely related to the same reaction for alkenes, and one catalyst that is specific to alkynes was introduced by Schrock (who with Grubbs won the Nobel prize for these discoveries) and is based on tungsten (M=W(OR)3).
In the previous post, I expressed surprise at the nature of the transition state for the alkene reaction, since the C-C or M-C bond (M=Ru) had hardly started to form by the time the transition state was reached. So what of the nature of the alkyne analogue? Firstly, I should mention that since the intention is to study the intrinsic reaction coordinate, which can be a lengthy calculation, it is necessary to prune the catalyst itself down to bare essentials. Unfortunately, it is now increasingly recognised that those sterically bulky groups that often adorn modern catalysts can in fact dramatically affect the nature of transition state. For one example where e.g. addition of bulky groups can transform a transition state to a minimum, see here[1] (and there may well be other examples of the reverse transformation of changing a minimum into a transition state). So with that caveat in place, take a look at the computed transition state (ωB97XD/Def2-SVPD/SCRF=dichloromethane) for a model where the t-Butoxy ligands of the real catalyst are replaced by simple OH groups.
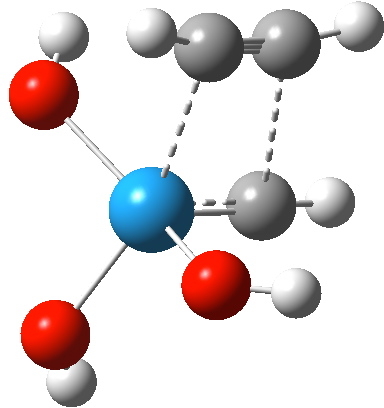
Transition state for addition of alkyne to Schrock catalyst. Click for 3D
The C-W and C-C forming bonds are respectively 2.45 and 2.59 Å long; both are significantly shorter than those for the alkene transition state. The latter however had four ligands other than the incoming alkene to reorganise, whereas this one has one fewer. With less reorganisation needed, it can start forming bonds earlier.
The transition state leads to a fascinating product, being a metallacyclobutadiene. The carbocycle itself is of course famously transient and unstable (normally ascribed to its being anti-aromatic). In contrast, changing one carbon to tungsten makes the ring stable enough to be isolated as a crystal, one example of which is shown below (with an imine replacing one of the alkoxy groups).
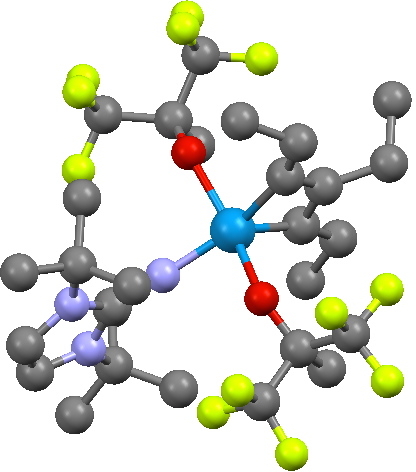
Crystal structure of a metallacyclobutadiene. Click for 3D.
This is an example where replacing an atom carrying a pπ orbital with one carrying a dπ orbital inverts the aromaticity rules. However, the alternating bond pattern characteristic of cyclobutadiene (and anti-aromaticity) remains visible in this structure. Thus the two C-C lengths in the X-ray structure below are 1.39 and 1.53Å, which perhaps corresponds to something like the following resonance form rather than implicating anti-aromaticity. More analysis is clearly needed here at some future stage. At any rate, the barrier for converting one bond-localized form into the other (via TS2) looks likely to be very small, and that the rate-determining-step is going to be TS1/TS1′.
The intrinsic reaction coordinate appears thus, with the C-C forming only shortly after the W-C bond is formed.
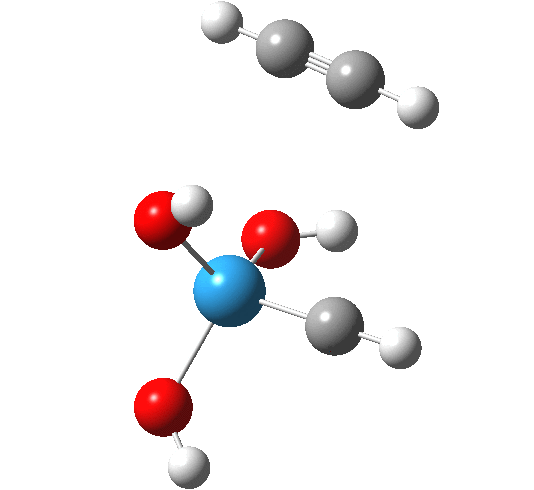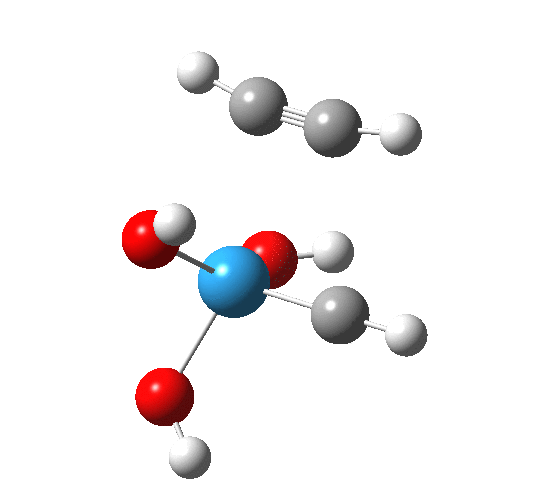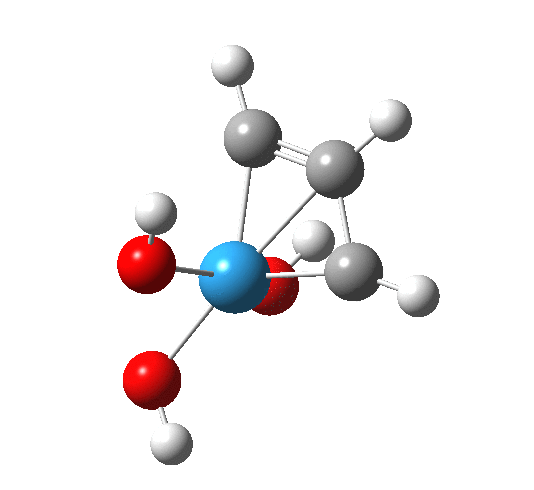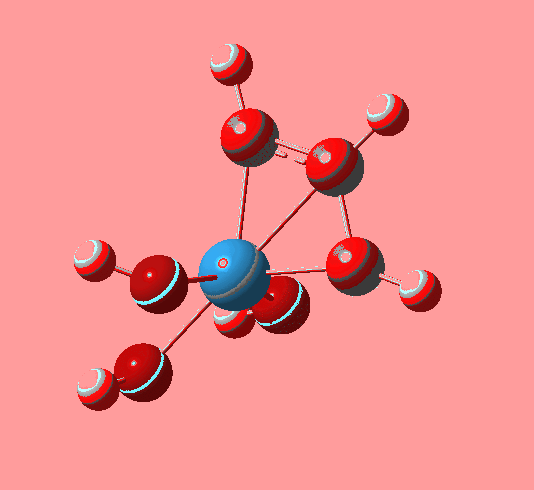
A postscript to the above. The IRC paths for these reactions are particularly difficult to compute; the secret lies in discovering the correct combination of parameters and step size to use. As a result, I have been able to chart a larger proportion of the IRC than initially reported.
- The barrier to addition of ethyne is smaller than found previously for alkene
- At IRC -5, we start to see several features (amplified in the gradient norm along the IRC) which correspond to conformational reorganisation of the hydroxyl groups attached to the metal
- The final conformation of these matches the crystal structure shown in the post. This leads one to conclude that the conformation of these ligands may be crucial in determining the catalytic activity of the system
- In the final conformation, the two C-C bond lengths are predicted as 1.41Å and 1.45Å. The crystal structure shows a rather greater asymmetry, but perhaps we can see the origins of this asymmetry as originating in the conformational re-orientation of the di-axial alkoxy groups. If you look at the IRC very carefully, you will notice that near the end, the W-OH groups start to strongly rotate. As they do so, the relative lengths of the two C-C bonds invert (ie the longer one ends up as the shorter one). This in turn implies that the orientation of the lone pairs on the oxygen controls the relative lengths of the two C-C bonds of the metallacyclobutadiene.
- So the IRC in the end teaches us some very interesting stereoelectronic features of this catalytic system which deserve to be further investigated.
So this brief foray into metathesis chemistry seems to indicate that the attributes of the alkene and alkyne reactions are indeed rather different, most obviously in the amount of reorganisation in the ligand coordination geometry that each requires. The full story however is bound to only emerge when realistically sized ligands replace the simple small ones here.
References
- K. Abersfelder, A. Russell, H.S. Rzepa, A.J.P. White, P.R. Haycock, and D. Scheschkewitz, "Contraction and Expansion of the Silicon Scaffold of Stable Si<sub>6</sub>R<sub>6</sub> Isomers", Journal of the American Chemical Society, vol. 134, pp. 16008-16016, 2012. https://doi.org/10.1021/ja307344f
Tags: Reaction Mechanism, X-ray