The Curtius reaction is represented in most chemistry texts and notes as following path (a) below. It is one of a general class of thermally induced rearrangement which might be described as elimination/migration (in a sense similar to this ring contraction migration/elimination), in this case implicating a nitrene intermediate if the two steps occur consecutively. Wikipedia is normally very much on the ball with this sort of thing, and a comment about the reaction mechanism there notes that current evidence prefers route (b), avoiding nitrene intermediacy (and hence formally removing this from examples of nitrene reactions).
So time for a reality check (which in this case takes the form of a ωB97XD/6-311G(d,p)/SCRF=dichloromethane calculation).
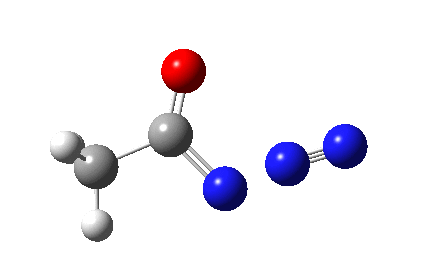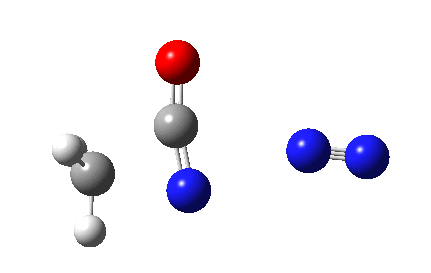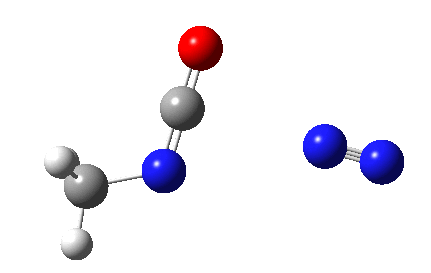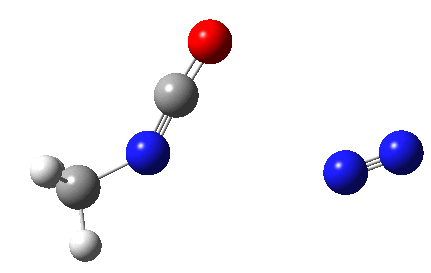
This is pretty clear-cut; no nitrene intermediate. Now for the standard text-books to catch up!
Tags: elimination, migration, pericyclic, Reaction Mechanism, Tutorial material