The anti-periplanar principle permeates organic reactivity. Here I pick up on an example of the antiperiplanar E2 elimination (below, blue) by comparing it to a competing reaction involving a [1,2] antiperiplanar migration (red).
The relative rates of these two processes will depend on several factors such as the ability of Cl to donate electrons (red) vs the basicity of the chloride anion (blue) and of course solvent polarity. It is the balance between these two mechanisms that caught Barton’s eye and helped him formulate his ideas about conformational analysis. Calculations (ωB97XD/6-311G(d,p)/SCRF=water) help reveal the basic features of the competition; details can be found for the ring contraction and E2 elimination.
| Ring contraction | E2 Elimination |
|---|---|
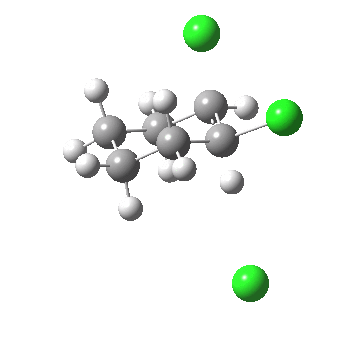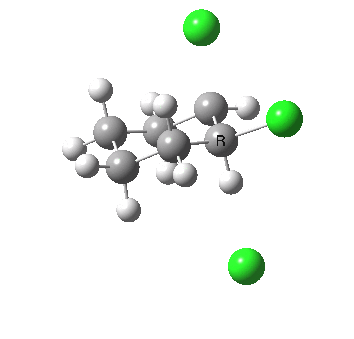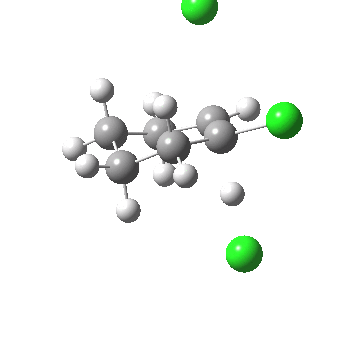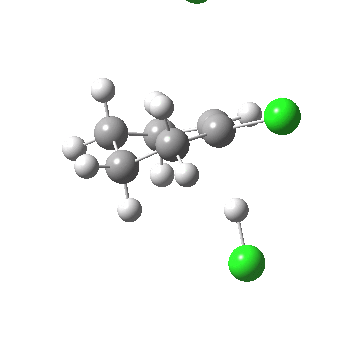
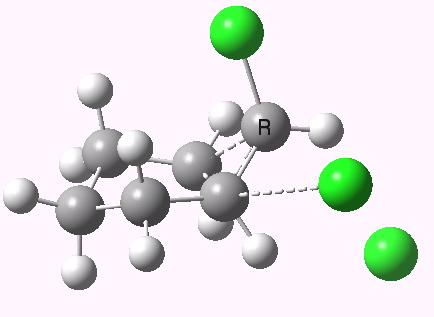 Ring contraction. Click for 3D. |
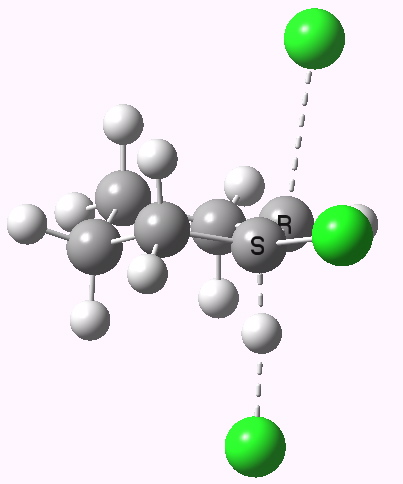 E2 elimination. Click for 3D. |
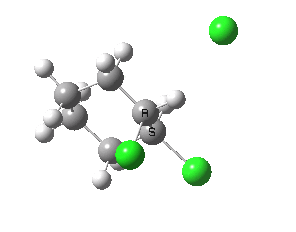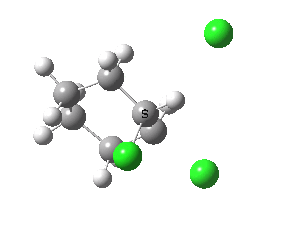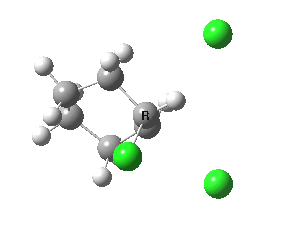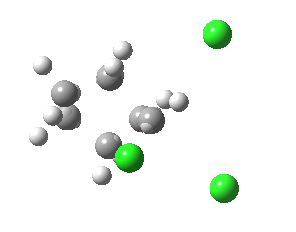
If you focus on the dashed bonds, you can easily identify the anti-periplanar components for each reaction. In this specific example, the E2 reaction wins out over the ring contraction/migration by ΔΔG298 = 17.6 kcal/mol. (One of) the orbital interactions responsible for the antiperiplanar migration is shown below.
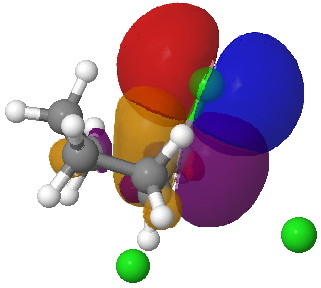
The orbital overlap in the app migration. Click for 3D
 |
 |
You might notice that via these posts, I am gradually building up a library of transition states for taught reactions. Still a few to go however!
[…] induced rearrangement which might be described as elimination/migration (in a sense similar to this ring contraction migration/elimination), in this case implicating a nitrene intermediate. Wikipedia is normally very much on the ball with […]