In the previous post, I discussed what we could learn from ethane by forcing it into a pericyclic dyotropic rearrangement. We saw how it voraciously scavenged two electrons from the C-C bond to achieve this. What if we give it more electrons? Thus 1,2-dibromoethane undergoing the same reaction.
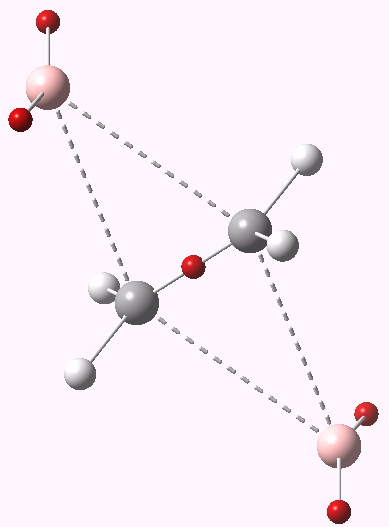
Subjected to B3LYP/6-311G(d,p) (with or without solvent field) yields a transition state with only one negative force constant. No tendency to distort from D2h symmetry then. Notice also how the migrating hydrogens did all the moving for ethane, but with a much heavier bromine replacing them, it is now the relatively light carbons and the hydrogens attached to them that instead carry the reaction.

Transition state for dyotropic rearrangement. Click for 3D.
This shows that the C-C region has 2.4 electrons; its actually gained some! The bromines each have 7.9 electrons in the valence shell in the form of two lone pair monosynaptic basins (and 27.5 in the core), and the remaining hydrogens on the carbon have ~2.15 each (0.15 having been ~extracted from the core of the bromine). The system has distorted from a pericyclic transfer of electrons to an ionic mechanism; an ion-triple to be precise. This is also not anti-aromatic. So here we see yet another way in which a forbidden (anti-aromatic) pericyclic reaction can distort. There are other ways still!
Tags: dibromoethane, dyotropic, iontriple, pericyclic, Tutorial material

[…] So, ethane taught us a lot in the end. In trying to persuade it to undergo a dyotropic rearrangement, we found it came across the undesired antiaromaticity in the transition state. A nice simple example of a pericyclic selection rule in action. Of course, one could make things more complicated, but I leave that to another day. […]
[…] Henry Rzepa Chemistry with a twist « More is more: the dyotropic rearrangement of 1,2-dibromoethane. […]
The above post caused this crystallographer a wry smile as this mechanism for swapping the peripheral heavy atoms co-incidentally exactly matches a commonly observed disorder for this type of unit in crystal structures. Usually disorder happens more with peripheral atoms (the ones that have more degrees of freedom) but when the peripheral atoms are heavy and in the right geometry one can get situations like this where the peripheral atoms “stay still” and it is the backbone that is disordered. I had a recent case of a disordered 1,2-dichloroethane solvent molecule that had exactly this with one position for the chlorine atoms and two for the central ethane unit.