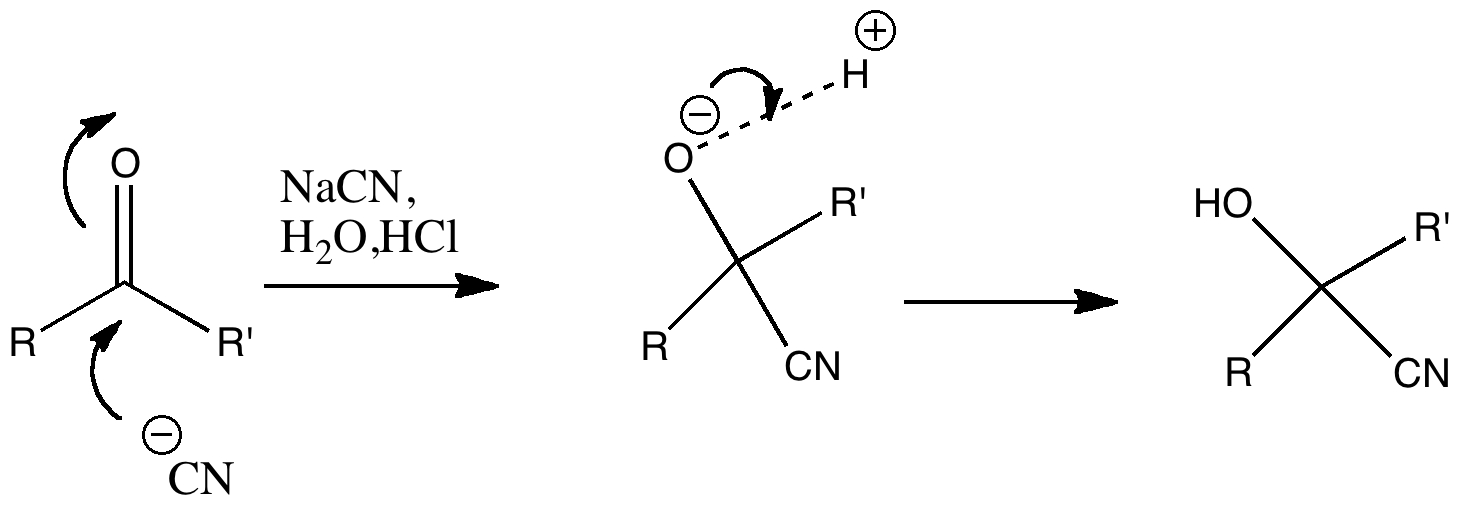
Nucleophilic addition of cyanide to a ketone or aldehyde is a standard reaction for introductory organic chemistry. But is all as it seems? The reaction is often represented as below, and this seems simple enough.
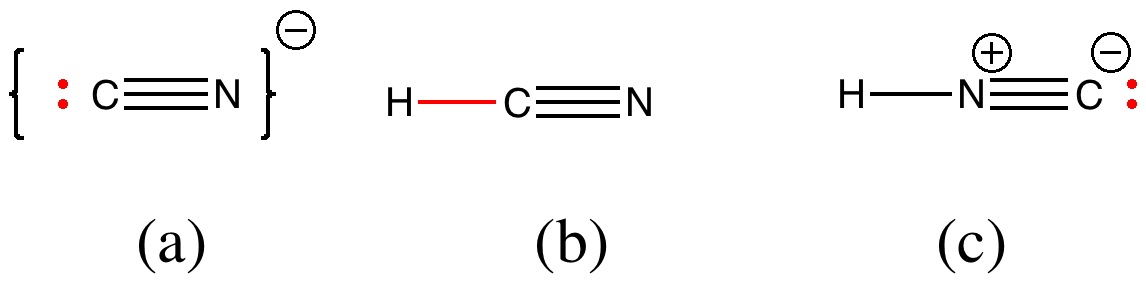
But attention to detail suggests that, HCN being a weak acid, there will be only a very small concentration of cyanide anion in the presence of HCl. There are other aspects which (if fussy) one might quibble with. The arrow pushing originates at the negative sign of the cyanide group. It is slightly more accurate to suggest that any arrow shown originates at an electron pair rather than necessarily a charge (think borohydride anion).
In cyanide anion, the relevant electron pair is shown above in red (a). But if most of this anion is really protonated in HCl, then the electron pair resides in a H-C bond (b). Or, if we put the proton on the nitrogen, then again it becomes a lone pair (c). So, can we formulate a mechanism for cyanohydrin formation for acidic solutions, which avoids the need to use (a)?
We need to borrow a water molecule, and then isomerise the HCN to HNC, before subjecting the combination to a cyclic reaction. Is this viable? To answer that, we will do a ωB97XD/6-311G(d,p)/SCRF=water calculation. Solvating this reaction with both (at least) one explicit water, and a continuum field model is crucial. The calculated free energy of activation for this process with respect to HCN+H2O+carbonyl is 30.0 kcal/mol. This is a bit high for a reaction that occurs readily at room temperatures, but perhaps with a better model which includes more explicit water molecules, it might be regarded as a reasonable alternative to the cyanide anion mechanism.
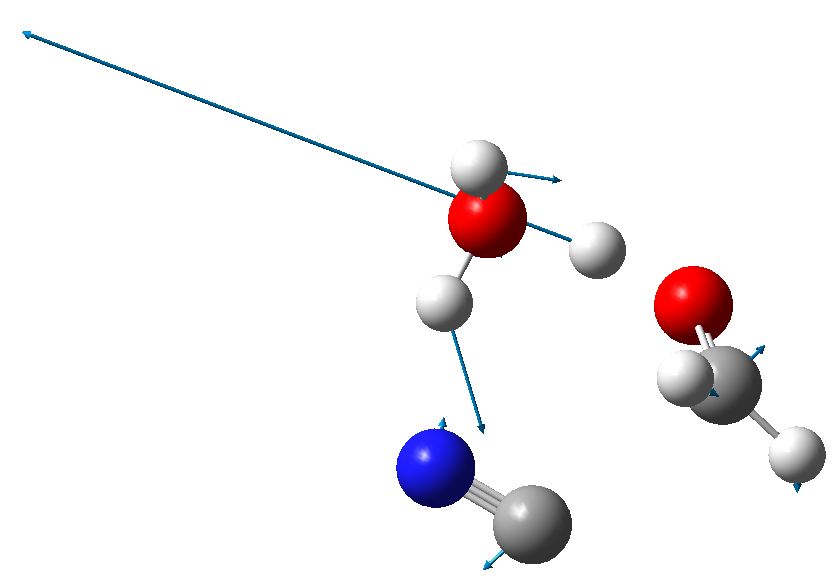
Transition state for cyanohydrin formation. Click for 3D
This results in a 6-ring transition state with an activation free energy of 33.4 kcal/mol with respect to HCN+H2O+protonated carbonyl. This transition state has a very unusual feature, namely water acting as a base removing the proton from HCN, and the same carbon that is losing this proton is also forming a new C-C bond to make the cyanohydrin. Such a bimolecular displacement at an sp-hybridized carbon centre is quite unusual (and it also happens with retention of “configuration” at the carbon, an SNi reaction). Notice also that the proton removal occurs as a linear geometry, and the carbon attacks the (protonated) carbonyl at 111°.
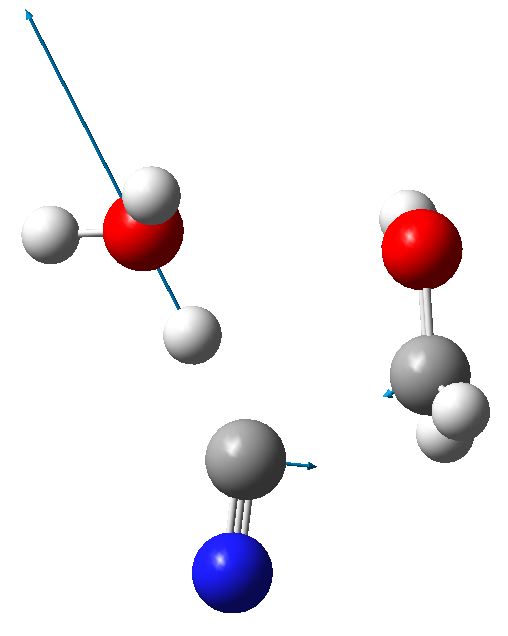
6-ring transition state. Click for 3D
Tags: acidic solutions, activation free energy, calculated free energy, cyanohydrin, mechanism, Tutorial material
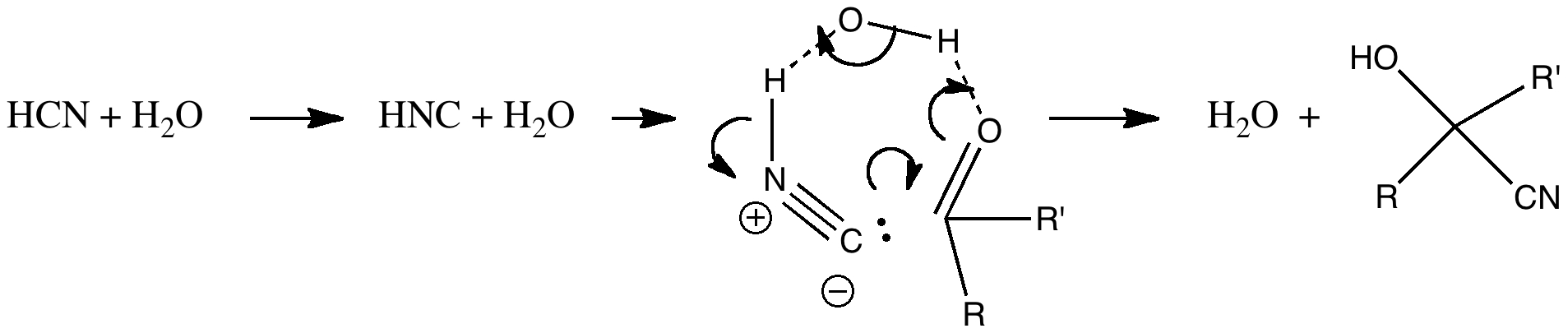
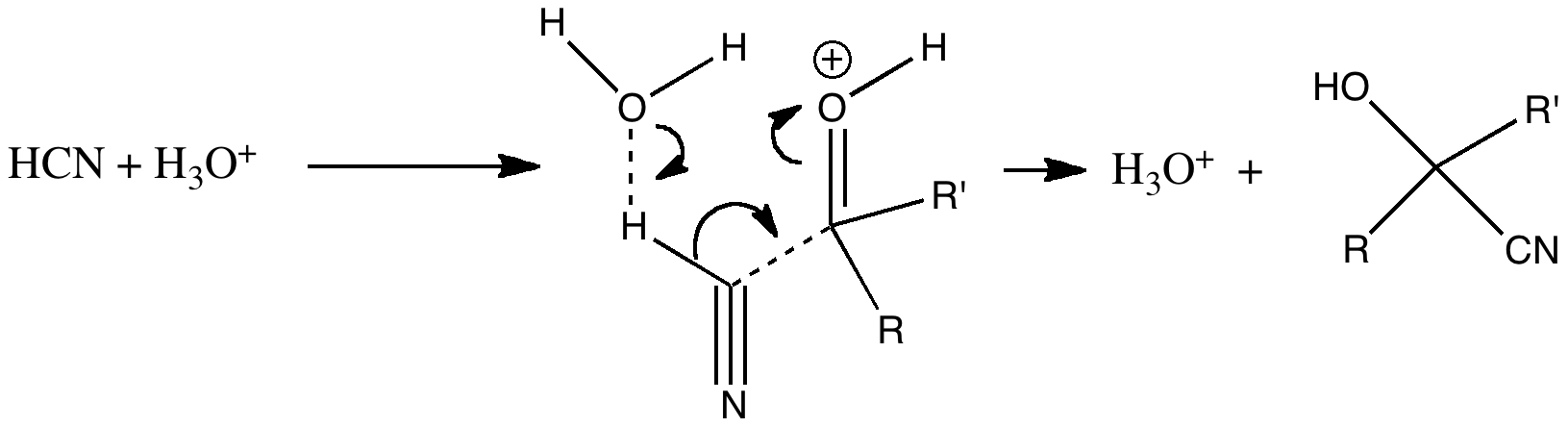
The above discussion seems to indicate that under conditions where very little cyanide anion is present, the formation of cyanohydrin has a kinetic barrier of 30 kcal/mol or more. In other words a very slow thermal reaction.
In fact, if one consults a classic article by Arthur Lapworth (doi: 10.1039/CT9038300995, one finds the statement (p 996, Mineral acids in small quantities were found to prolong the time occupied to a very marked extent, and with one drop of ordinary hydrochloric acid … no noticeable diminution in colour (i.e. reaction) had taken place at the end of 14 days. This agrees remarkably well with the thermal barrier of > 30 kcal/mol as computed above.
Lapworth goes on to state Small quantities of bases, on the other hand, produced a very great acceleration.
This requirement for the absence of any strong acid for the reaction to occur is perhaps not emphasised sufficiently. The take-home lesson is that: