One of the delights of wandering around an undergraduate chemistry laboratory is discussing the unexpected, if not the outright impossible, with students. The >100% yield in a reaction is an example. This is sometimes encountered (albeit only briefly) when students attempt to recrystallise a product from cyclohexane, and get an abundant crop of crystals when they put their solution into an ice-bath to induce the crystallisation. Of the solvent of course! I should imagine 1000% yields are possible like this.
What the students are not expecting is that cyclohexane has such a high melting point, higher than that of water! n-Octane for example melts at -57°C (and most of us have seen those travelogues in the antarctic where the petrol tanks need to be warned to prevent freezing), so why is that of cyclohexane so much higher? That it might be strange is shown by the melting points of the series:
- benzene, +5.5°C
- cyclohexadiene, -89°C
- cyclohexene, -97°C
- cyclohexane, +6.5°C.
Benzene one might explain because it famously stacks in a herring-bone fashion, with the relatively electropositive hydrogen attracted to the π-cloud on the face.

The crystal structure of benzene. Click for 3D
Clearly, this explanation cannot hold for cyclohexane, which has no π-face. What does the crystal look like?
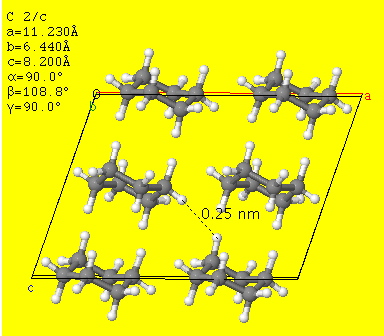
Crystal structure of cyclohexane. Click for 3D
If one inspects the structure closely, one can find quite a few H…H contacts at about 2.4Å and they are arranged in a particularly rigid three-dimensional manner. The maximum attractive force resulting from van der Waals, or dispersion interactions between two hydrogens is thought to occur at ~2.4Å. Perhaps cyclohexane is a prime (possibly THE prime) example of the influence of this (under-rated) interaction? A molecule covered in Velcro no less. By the way, can you spot the connection with the previous post?
Postscript: Below is a so-called non-covalent-analysis (NCI) of cyclohexane as packed into a crystal lattice. The coordinates are obtained from a neutron diffraction structure. The green regions indicate weakly attractive zones.
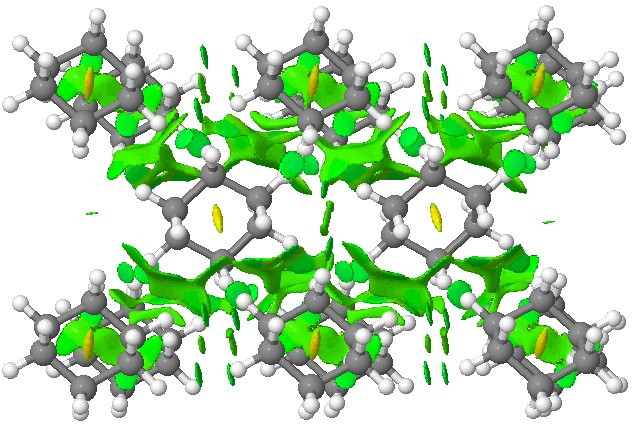
Click for 3D.
Tags: antarctic, benzene, cyclohexane, dispersion interactions, melting points, Postscript, Tutorial material
Very nice post. I am not sure if you are aware, but Grimme published a nice paper in 2008 (link below) regarding (among other things) the similarity of non-covalent interactions between “stacked” benzene dimers and cyclohexane dimers: 10.1002/anie.200705157
In the present context, it’s unclear to me why cyclohexene and cyclohexadiene can not adopt similar “stacked” arrangements. I will have to think about that for a bit.
Thanks Steven for pointing this out. I suspect that since benzene has a fairly strong (non-induced) permanent quadrupole moment, there must be a significant electrostatic contribution from that term. Dispersion forces are supposed to arise from instantaneous induced dipoles (why not also induced quadrupoles?). Are these not of a different nature?
I have found another interesting application of dispersion forces in DNA to be found in this post, which might play a role in the preference of a CG-rich duplex to adopt the Z-helical form.
Here is another series, the melting points of the cycloalkanes from n=3 to n=10; -127, -90, -94, +6.5, -13, +14, +10, +10. The jump from cyclopentane to cyclohexane is very striking!
Sorry, Henry, but crystal packing, rather than dispersion differences are the main reasons for these remarkable m.p. variations. Dispersion differences should influence the b.p’s of these HC’s as well, but they are all the same! (in deg C)
benzene 80.1
1,3-cyclohexadiene 80.5
1,4-cyclohexadiene 82
cyclohexene 83.9
cyclohexane 80.7