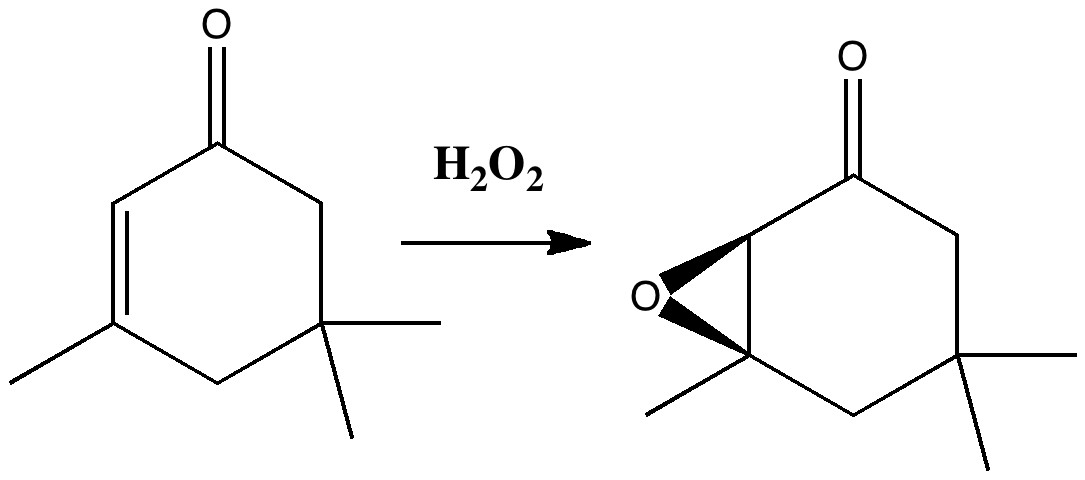
Our understanding of science mostly advances in small incremental and nuanced steps (which can nevertheless be controversial) but sometimes the steps can be much larger jumps into the unknown, and hence potentially more controversial as well. More accurately, it might be e.g. relatively unexplored territory for say a chemist, but more familiar stomping ground for say a physicist. Take the area of asymmetric synthesis, which synthetic chemists would like to feel they understand. But combine this with gravity, which is outside of their normal comfort zone, albeit one we presume is understood by physicists. Around 1980, one chemist took such a large jump by combining the two, in an article spectacularly entitled Asymmetric synthesis in a confined vortex; Gravitational fields and asymmetric synthesis[1]. The experiment was actually quite simple. Isophorone (a molecule with a plane of symmetry and hence achiral) was treated with hydrogen peroxide and the optical rotation measured.
Conventional wisdom is that the oxygen can be delivered with equal probability to either face of the alkene, resulting in a racemic (equal) mixture of the two enantiomers of the epoxide. But if one enantiomer is formed in slightly greater amount than the other, the reaction is said to proceed asymmetrically, and the product will exhibit an optical rotation. Normally, such asymmetry is induced by carrying out the reaction in the presence of a chiral molecule or catalyst. Light too can be chiral, but these brave chemists decided to use gravity. More specifically, the earth’s gravitational field. In the Northern Hemisphere. The reaction was conducted in a centrifuge in three ways. With the spun tube horizontally, and then vertically spinning clockwise or anticlockwise. The first of these produced product which exhibited no optical rotation within experimental error (e.g +0.2 ± 0.3 mdeg). The second gave results with a positive rotation (e.g. 12.8 ± 0.3 mdeg) and the third a negative rotation (-2.2 ± 0.2 mdeg). They considered that the reaction was occurring in e.g. a clockwise vortex constituting a P-helix (the vortex in other words was chiral) interacting with the earth’s coriolis force. They speculated (but did not do the experiment) that the reverse effect would be seen in the Southern Hemisphere. Their paper concluded with the grand speculation that prebiotic organic synthesis could have been partially asymmetric as a result of being conducted in a chiral gravitational field (nothing like aiming high!).
Shortly after this was published, a rebuttal appeared[2] penned not by a synthetic chemist but a physicist. In truth, most of the four-paragraph article presents arguments few chemists are familiar with (and probably do not understand). Only one sentence, the very last, made the impact (and it sounds as if it was added as a throw-away afterthought). It simply stated that the magnitude of the earth’s field (in so-called natural units) suggested that a parameter ω related to the spinning velocity was ~10-21 and that the corresponding value that would be required to induce the asymmetry observed (which can be computed from e.g. ΔG = -RT Ln K) was 10-14. Put in rather plainer English, the earth’s magnetic field was seven orders of magnitude too weak to have the effect claimed for it. That last sentence on its own pretty much sunk the theory, and it is no longer thought that gravitational fields can induce asymmetry in reactions. I tell this story (which, as it happened thirty years ago is now largely forgotten), since seven orders of magnitude is quite a large mismatch! Chemists rarely have the opportunity to be so spectacularly wrong when they propose a theory. In reality, even two orders of magnitude is unusual.
It’s when you approach factors of say less than one order of magnitude (the nuances) that arguments about which interpretation is correct may break out. So which category might the subject of this post belong to? As I there noted, it’s all about whether the two carbon atoms separating carbon dioxide and cyclobutadiene in a crystalline lattice by a distance of 1.5Å are interacting by a covalent bond, or a van der Waals attraction. In terms of energies, most chemists agree that these differ by around two orders of magnitude. This, I suggest, does not come into the category of nuance!
A nice review of asymmetric synthesis under physical field[3].
References
- R.C. Dougherty, "Chemical geometrodynamics: gravitational fields can influence the course of prochiral chemical reactions", Journal of the American Chemical Society, vol. 102, pp. 380-381, 1980. https://doi.org/10.1021/ja00521a067
- A. Peres, "Asymmetric synthesis in a spinning vessel", Journal of the American Chemical Society, vol. 102, pp. 7389-7390, 1980. https://doi.org/10.1021/ja00544a051
- M. Avalos, R. Babiano, P. Cintas, J.L. Jiménez, J.C. Palacios, and L.D. Barron, "Absolute Asymmetric Synthesis under Physical Fields: Facts and Fictions", Chemical Reviews, vol. 98, pp. 2391-2404, 1998. https://doi.org/10.1021/cr970096o
Tags: chemist, chiroptical, Interesting chemistry, physicist, synthetic chemist