The Science education unit at the ACS publication C&EN publishes its list of molecules of the year (as selected by the editors and voted upon by the readers) in December. Here are some observations about three of this year’s batch.
- Diberyllocene[1] with its unusual Be-Be bond has already beeen covered on this blog.[2], where I commented that lithioborocene should be possible to make as well.
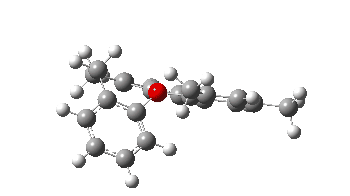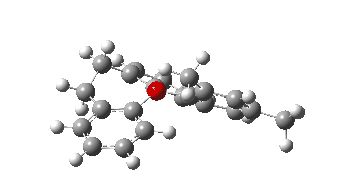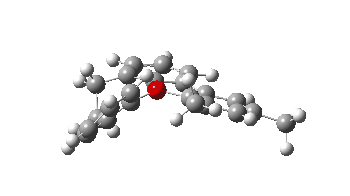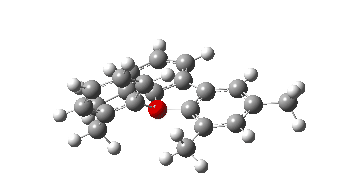
- The second in the list is the synthesis of the chiral triaryloxonium ion[3] HICBUU(Crystal DOI: 10.5517/ccdc.csd.cc2cjynj). Curiously, this combines the features of two of our recent publications (with Chris Braddock)[4],[5]. The first of these speculated upon a mechanism involving an intermediate (and as it happens chiral) oxonium ion and its subsequent rapid fragmentation by nucleophilic attack. This meant it was never isolated, unlike the one reported this year.[3] The second article of ours involved another class of chiral natural product called polysiphenols and their enantiomerisation by a process called atropisomerism via a two stage process. This as it happens is also the feature reported for the the chiral triaryloxonium ion.
The atropisomerism involves restricted (high energy) rotation about an axis shown as a dotted red line, accompanied at a separate stage with rotation about the C-C single bond shown in blue. The polysiphenols showed similar two-stage atropisomerism.
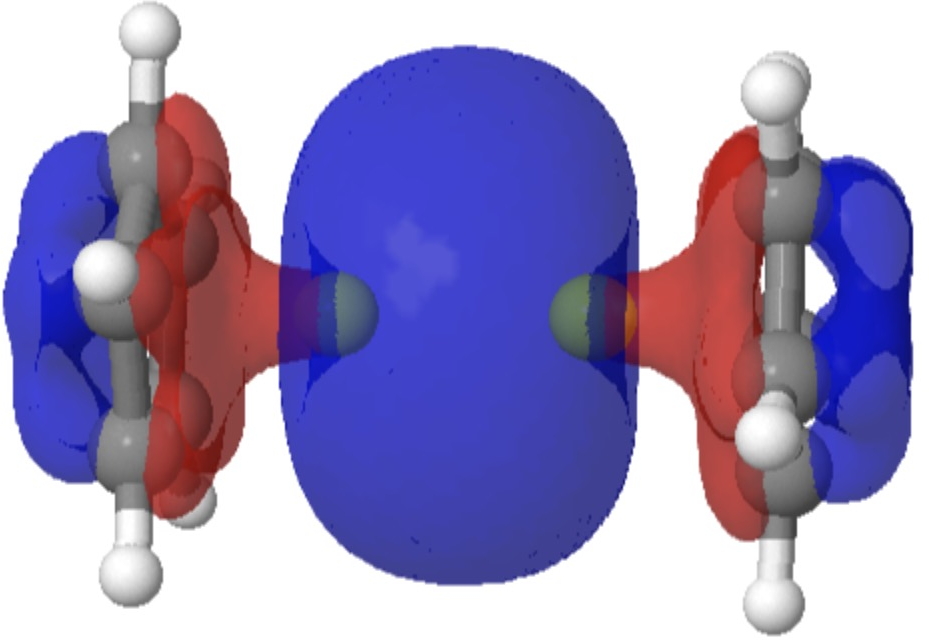
I show an intrinsic reaction coordinate calculation of this process below, being respectively the energy response and the dihedral angle responses about the 16-17 bond (blue) and the axis 2-18 (dashed red) and ending with an animation of the process.
Note how the C-C rotation is much lower in energy than the about the red axis. It is also interesting to observe that pyramidal inversion at the chiral oxygen centre via a trigonal planar unit only happens at an IRC value of ~+30, well after both transition states have passed!
- The third topic is represented by the crystal structure NITRUH (Crystal DOI: 10.5517/ccdc.csd.cc2fcr2n),[6]. This is announced at the C&EN page as “carbene breaks octet rule”, in having only four valence electrons at the central carbon atom. The structure is certainly unique, the motif shown below having only one entry in the crystal structure database. Leaving aside the observation that a true carbene also breaks the octet rule with its nominal six valence electrons, how could this arise? Well, shown below are six canonical forms of this species (the original article shows four) of which species 1a is the one referred to as having only four valence electrons at the central atom, whilst 1b is that favoured in the article.
What does a calculation reveal (ωB97XD/Def2-TZVPP; DOI for data 10.14469/hpc/13532)? The Wiberg bond index calculated for the central carbon atom is 3.8328, which corresponds to 7.67 electrons. Far from four! The Wiberg bond orders along the chain are 1.54, 1.14, 1.83, 1.83, 1.12, 1.58 (the species is calculated as not quite symmetrical as an isolated molecule) which is a close match to structure 1e (not shown in the article). Dare I suggest that the tag line for this entry in the C&EN article is a good example of copywriters hyperbole, something designed to catch the interest and attention of the reader? Well, it certainly succeeded, but I venture to suggest that although the molecule is indeed interesting and unique, it does NOT break the octet rule. A good discussion point perhaps for a chemistry tutorial?
References
- J.T. Boronski, A.E. Crumpton, L.L. Wales, and S. Aldridge, "Diberyllocene, a stable compound of Be(I) with a Be–Be bond", Science, vol. 380, pp. 1147-1149, 2023. https://doi.org/10.1126/science.adh4419
- H. Rzepa, "Diberyllocene — and Lithioborocene?", 2023. https://doi.org/10.59350/v1cma-xjk91
- O. Smith, M.V. Popescu, M.J. Hindson, R.S. Paton, J.W. Burton, and M.D. Smith, "Control of stereogenic oxygen in a helically chiral oxonium ion", Nature, vol. 615, pp. 430-435, 2023. https://doi.org/10.1038/s41586-023-05719-z
- J. Clarke, K.J. Bonney, M. Yaqoob, S. Solanki, H.S. Rzepa, A.J.P. White, D.S. Millan, and D.C. Braddock, "Epimeric Face-Selective Oxidations and Diastereodivergent Transannular Oxonium Ion Formation Fragmentations: Computational Modeling and Total Syntheses of 12-Epoxyobtusallene IV, 12-Epoxyobtusallene II, Obtusallene X, Marilzabicycloallene C, and Marilzabicycloallene D", The Journal of Organic Chemistry, vol. 81, pp. 9539-9552, 2016. https://doi.org/10.1021/acs.joc.6b02008
- D.C. Braddock, A. Duran-Corbera, M. Nilforoushan, Z. Yang, T. He, G. Santhakumar, K.A. Bahou, H.S. Rzepa, R. Woscholski, and A.J.P. White, "(±)-Polysiphenol and Other Analogues via Symmetrical Intermolecular Dimerizations: A Synthetic, Spectroscopic, Structural, and Computational Study", Journal of Natural Products, vol. 85, pp. 2650-2655, 2022. https://doi.org/10.1021/acs.jnatprod.2c00749
- Y.K. Loh, M. Melaimi, M. Gembicky, D. Munz, and G. Bertrand, "A crystalline doubly oxidized carbene", Nature, vol. 623, pp. 66-70, 2023. https://doi.org/10.1038/s41586-023-06539-x