Respiratory pigments are metalloproteins that transport O2, the best known being the bright red/crimson coloured hemoglobin in human blood. The colour derives from Fe2+ at the core of a tetraporphyrin ring. But less well known is blue blood, and here the colour derives from an oxyhemocyanin unit based on Cu1+ (the de-oxy form is colourless) rather than iron. See below for the carapace of a red rock crab.
 Here I take a look at this very unusual structure, the core of which is an imidazole ring coordinated via nitrogen to the metal Cu.
Here I take a look at this very unusual structure, the core of which is an imidazole ring coordinated via nitrogen to the metal Cu.
A search of the crystal structure database for the following sub-structure
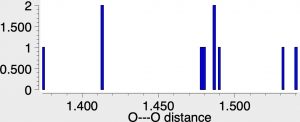
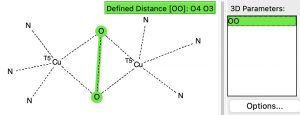 reveals 12 hits, with a range of O-O distances ranging from 1.37 to 1.54Å. A histogram of the O-O lengths in the Cu(O-O)Cu sub structure shown below shows quite a distribution amongst the 12 known examples.
reveals 12 hits, with a range of O-O distances ranging from 1.37 to 1.54Å. A histogram of the O-O lengths in the Cu(O-O)Cu sub structure shown below shows quite a distribution amongst the 12 known examples.
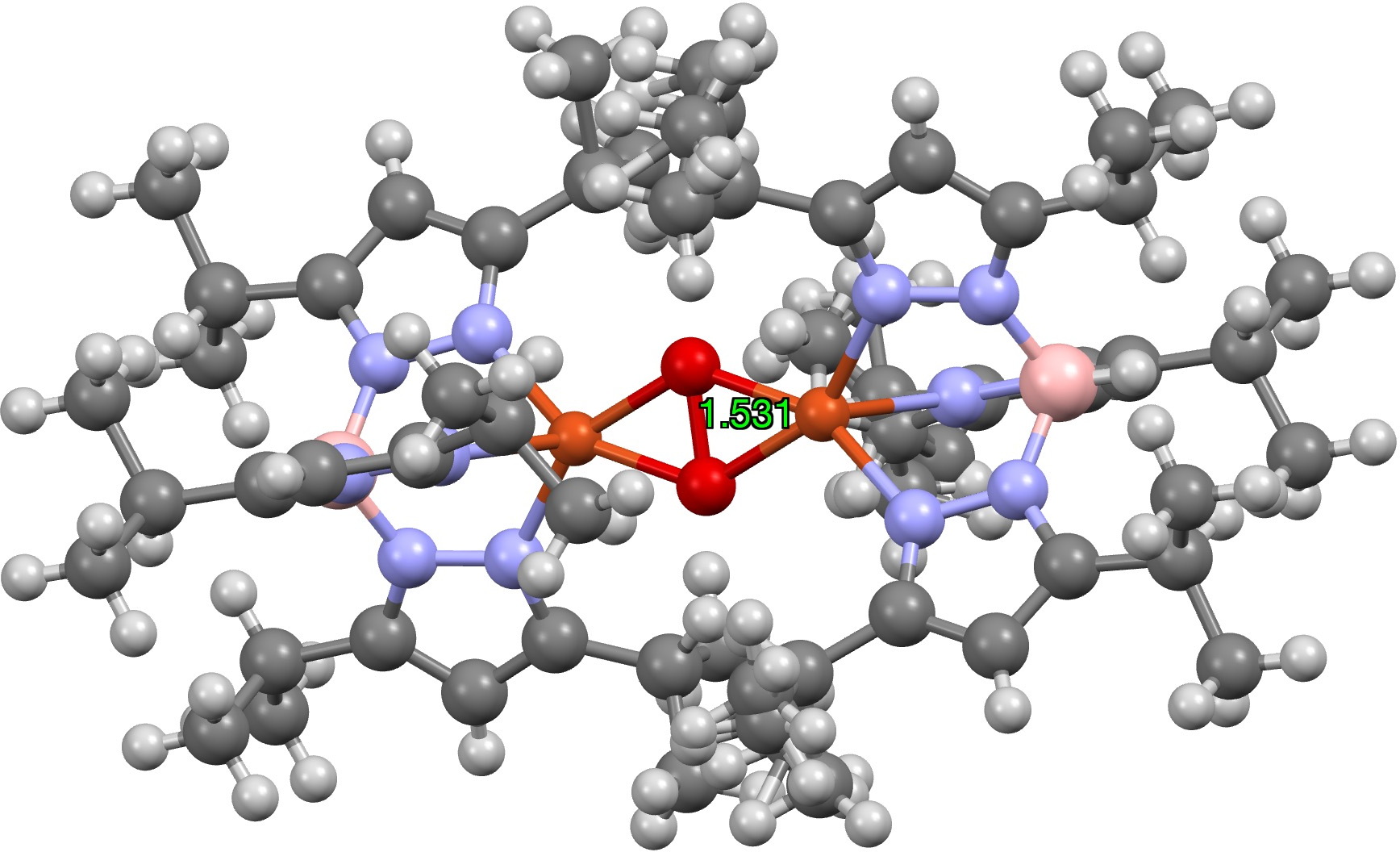
Of these, one (UTETEU[1], DOI: [2]) is perhaps the closest to the oxyhemocyanin core, albeit with the imidazole heterocycle replaced by the isomeric pyrazole ring (no Ag or Au examples are known). The overall 2+ charge deriving from two Cu1+ units is internally balanced with two 4-coordinate B1- end caps, and this system was chosen as the starting model for some computational studies.[3]
Firstly, the crystal structure reveals an O-O distance of 1.531Å; the O=O distance (from crystal structures where it is present) is ~1.24Å (DOI: 10.5517/cct597h) for neutral (triplet?) oxygen, ~1.50Å for the dianion O22- and 1.32Å for the monoanion O21-[4].
Computational models were constructed at the ωB97XD/Def2-SVPP level, FAIR Data DOI: 10.14469/hpc/12584.
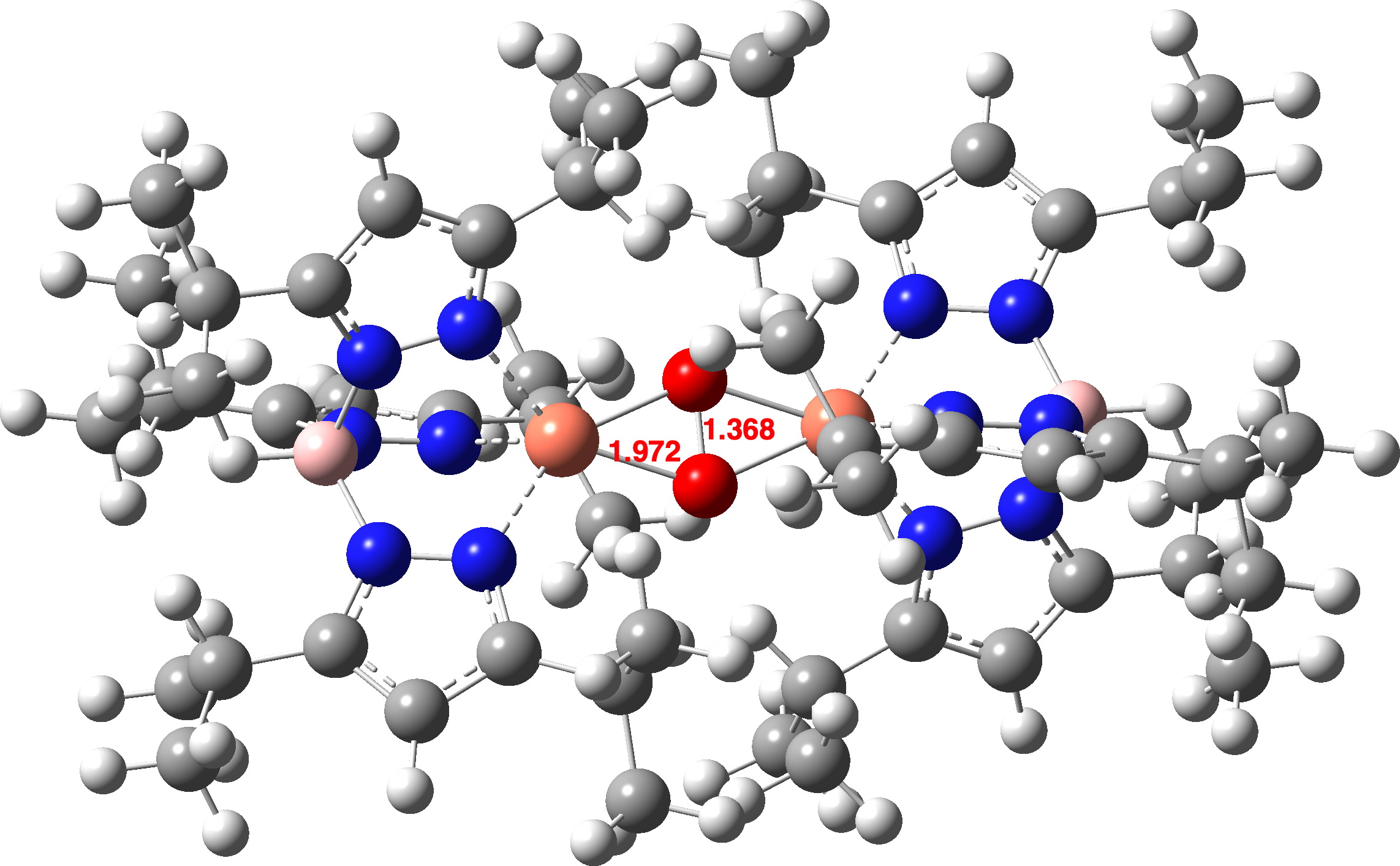
The computed O-O distance for a singlet state of the complex is shorter than that measured in the crystal structure (1.368 vs 1.531Å). At the better Def2-TZVPP basis set level, the O-O bond length is 1.379Å, still shorter. A model of singlet state oxyhemocyanin itself (Def2-TZVPP) as a di-cation (these charges are balanced by carboxylate anions from the surrounding protein) shows a very similar O-O bond length (1.361Å).
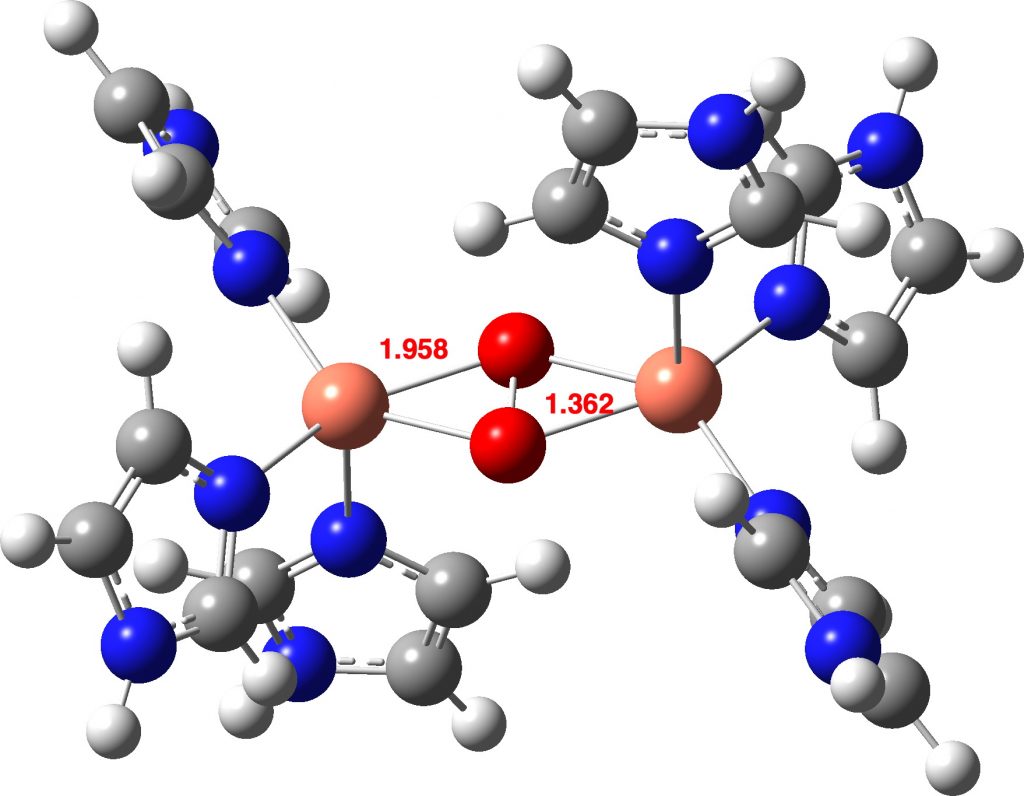
How about the oxyhemocyanin as a triplet state, the same state of isolated oxygen itself? Oxyhemocyanin now has a O-O distance of 1.477Å (Def2-TZVPP) and a Cu-O distance of 1.972 (1.934 from crystal structure of UTETEU). The UTETEU analogue has a calculated distance of 1.483Å (crystal structure 1.531Å), which strongly suggests that this system exists as a triplet rather than as a singlet spin state (click on image below to view as a 3D model).
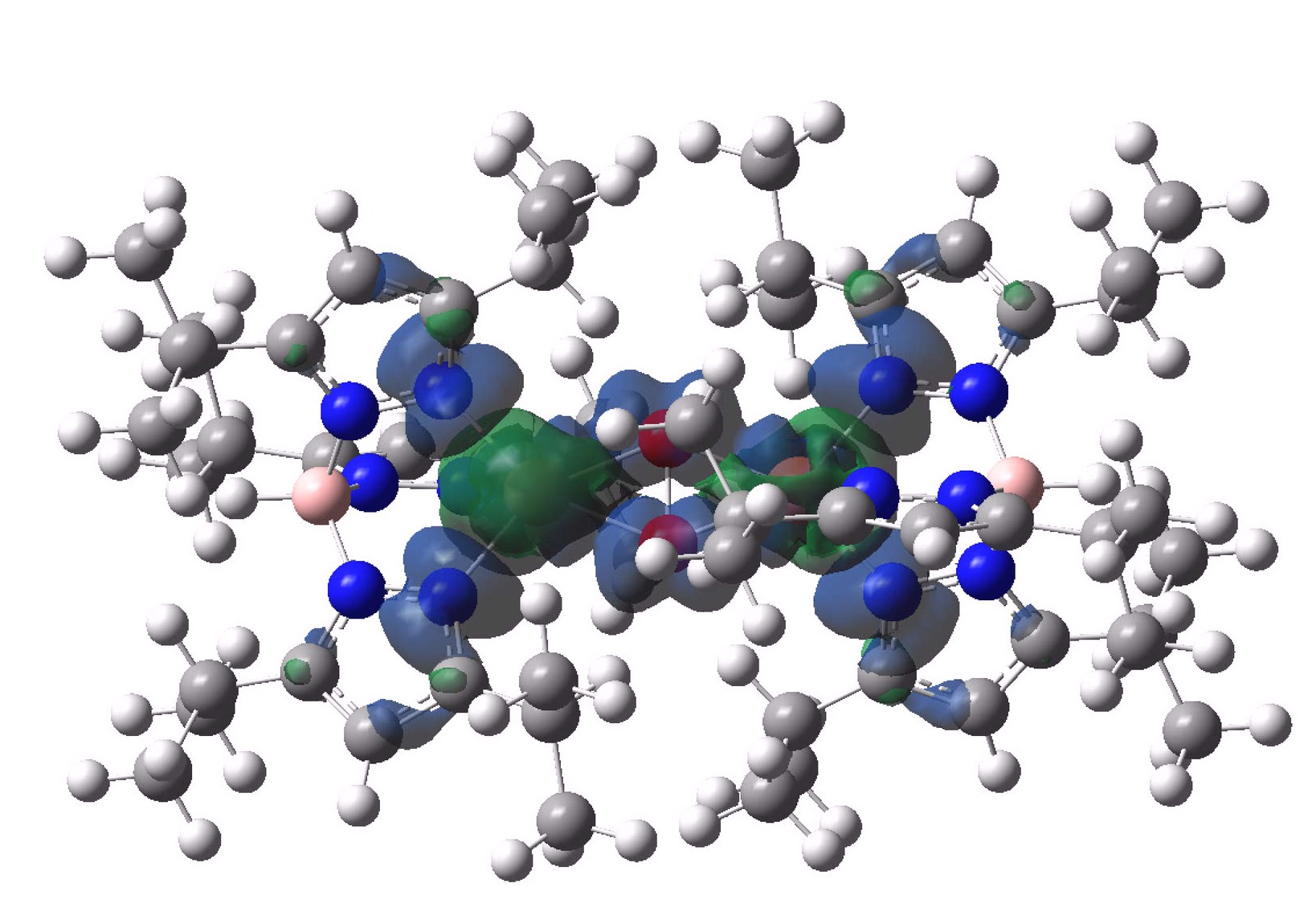
The spin density in UTETEU is shown above, which indicates that the two unpaired electrons are delocalised on Cu, nitrogen and O atoms, compared to only the oxygen in O2 itself.
So we may conclude from this brief investigation into the structures of this component of “blue blood” captures oxygen as a sandwich between two copper atoms (a mode very unlike the iron equivalent in hemoglobin), and moreover that the spin state in this capture retains the triplet motif of gaseous oxygen itself, whilst the spin density of the unpaired electrons is delocalised over both copper, nitrogen and oxygen.
This post has DOI: 10.14469/hpc/13111
References
- R. Dalhoff, R. Schmidt, L. Steeb, K. Rabatinova, M. Witte, S. Teeuwen, S. Benjamaâ, H. Hüppe, A. Hoffmann, and S. Herres-Pawlis, "The bridge towards a more stable and active side-on-peroxido (Cu<sub>2</sub><sup>II</sup>(µ-η<sup>2</sup>:η<sup>2</sup>-O<sub>2</sub>)) complex as a tyrosinase model system", Faraday Discussions, vol. 244, pp. 134-153, 2023. https://doi.org/10.1039/d2fd00162d
- Zhang, Shiyu., Fallah, Hengameh., Gardner, Evan J.., Kundu, Subrata., Bertke, Jeffery A.., Cundari, Thomas R.., and Warren, Timothy H.., "CCDC 1468787: Experimental Crystal Structure Determination", 2016. https://doi.org/10.5517/ccdc.csd.cc1l9d7j
- N. Kitajima, K. Fujisawa, C. Fujimoto, Y. Morooka, S. Hashimoto, T. Kitagawa, K. Toriumi, K. Tatsumi, and A. Nakamura, "A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the .mu.-.eta.2:.eta.2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = isopropyl and Ph)", Journal of the American Chemical Society, vol. 114, pp. 1277-1291, 1992. https://doi.org/10.1021/ja00030a025
- H. Seyeda, and M. Jansen, "A novel access to ionic superoxides and the first accurate determination of the bond distance in O2−", Journal of the Chemical Society, Dalton Transactions, pp. 875-876, 1998. https://doi.org/10.1039/a800952j
Tags: Interesting chemistry