The recently reported synthesis[1] of octafluorocubane established a sublimation point as 168.1–177.1°C (a melting point was not observed). In contrast, the heavier perfluoro-octane has an m.p. of -25°C. Why the difference? Firstly, the crystal structure is shown below, albeit as a dimer rather than a periodic lattice (click on image to obtain 3D coordinates).
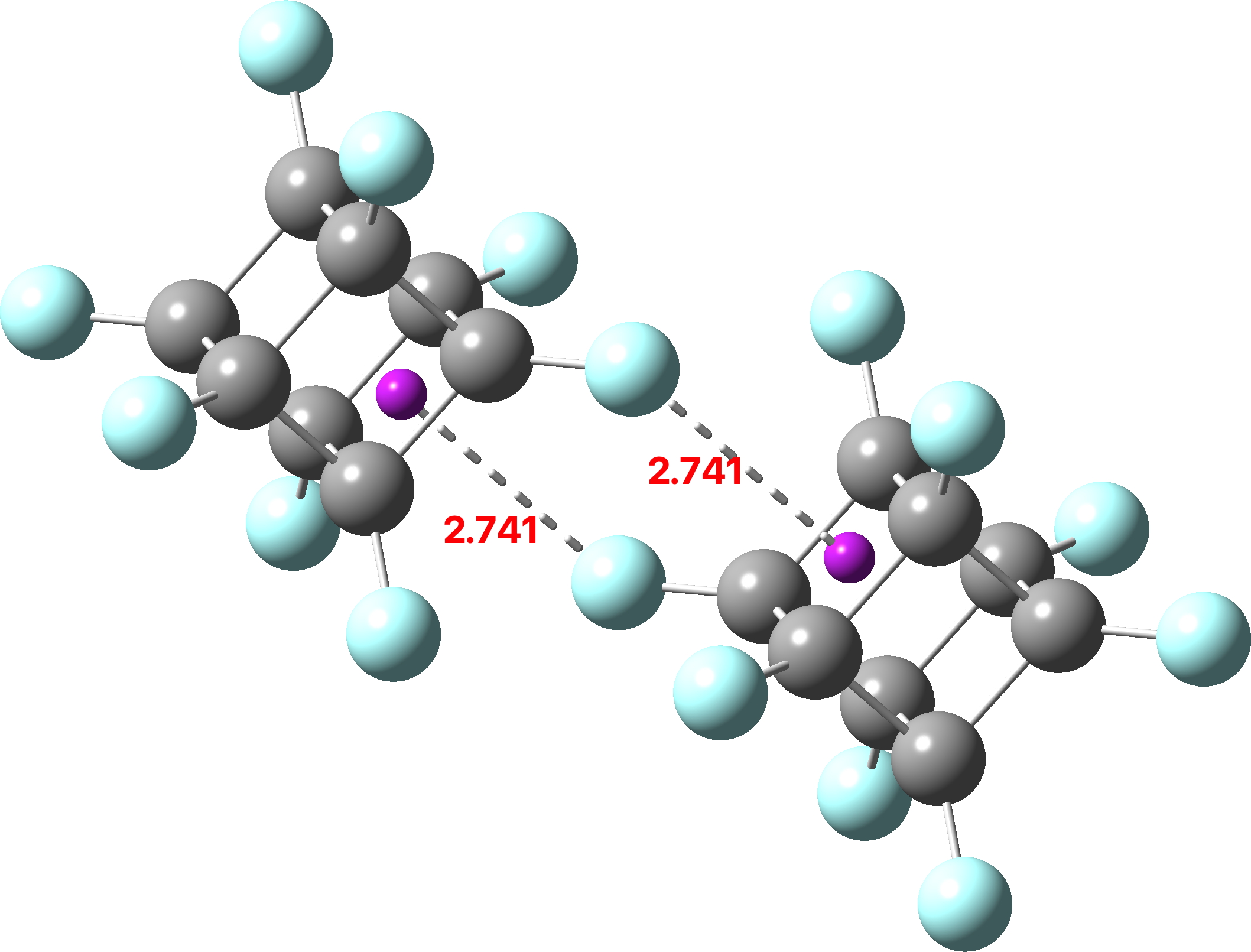
The distance between a fluorine and the centroid of the 4-membered carbon ring is 1.741Å. Our crystallographer (thanks Andrew!) gives me the following analysis of the periodic crystal lattice:
The asymmetric unit (crystal structure DOI: 10.5517/ccdc.csd.cc29z5p5) contains only two fluorine atoms (F1 and F2) and two carbon atoms (C3 and C4). Due to the symmetry/special positions, the C8F8 cube is formed of six C3’s, six F2’s, two C4’s and two F1’s. The 2.741Å contact comes from an F2 and hence there are six of these (and six faces). The closest F…C4(centroid) intermolecular separation for F1 is ca. 4.20Å. From the crystal structure one can indeed observe six C-F bonds of length 1.341Å and two of length 1.338Å, some 0.003Å shorter.
So time for some calculations (FAIR Data DOI: 10.14469/hpc/11132). The energies shown here are for the C2h-symmetric dimer relative to two monomers.
| Method | ΔE | ΔH | ΔG | F…centroid distance, Å |
| HF/Def2-TZVPP | -1.19 | -0.01 | +4.53 | 3.288 |
| B3LYP/Def2-TZVPP | -1.17 | -0.00 | +4.54 | 3.176 |
|
B3LYP+GD3+BJ/Def2-TZVPP |
-5.24 | -4.00 | +2.87 | 2.859 (2.741 expt) |
|
MP2/Def2-TZVPP |
-7.42 | – | – | 2.718 (2.741 expt) |
The three methods were chosen as approximations to establish (a) the effect of a dispersion/correlation correction, using the standard third-generation Grimme method and (b) the effect of more general dynamic correlations as being the difference between a Hartree-Fock calculation and DFT one. The values show the HFa and B3LYP-DFT as being very similar, but adding the GD3+BJ term stabilises the dimer significantly, as well as producing an F…centroid distance only a little longer than that measured. Each cube will sustain three pairs of such interactions, so the total stabilisation energy is ~15 kcal/mol and the enthalpy stabilisation is ~12 kcal/mol. A periodic boundary calculation of the complete cell would certainly be an even better model of this system. Nonetheless one further test, of the trend in length between the six interacting F atoms with a ring centroid and the two that do not (exp Δ-0.003Å shorter for the latter) is also replicated by the B3LYP+GD3+BJ/Def2-TZVPP calculation (Δ-0.006Å) which suggests the simple dimer model is not badly wrong.
So from these results, it appears that the attractive interactions between molecules octafluorocubane resulting in its high sublimation temperature may not be simply electrostatic interactions (a HF calculation would model that) or indeed of dynamic correlation (modelled by DFT methods) but a more complete electron correlation of the type normally described as dispersion and eg available via multi-reference and/or coupled-cluster methods. It may indeed come as a surprise that this molecule is a high melting solid because of dispersion, but the unique geometry allows an F to interact with four carbons via such forces, and to accumulate six of these per molecule in the crystal structure. So really quite unusual.
To end, it would certainly seem worthwhile to apply higher levels of theory to confirm this result, since the GD3+BJ induced-dipole/induced-dipole dispersion model is a relatively simple one, and as I commented in my WATOC notes, much higher level models of this effect are now becoming available.
aAs suggested by Cina Foroutan-Nejad, a commentator on the previous blog post
This post has DOI: 10.14469/hpc/11135
References
- M. Sugiyama, M. Akiyama, Y. Yonezawa, K. Komaguchi, M. Higashi, K. Nozaki, and T. Okazoe, "Electron in a cube: Synthesis and characterization of perfluorocubane as an electron acceptor", Science, vol. 377, pp. 756-759, 2022. https://doi.org/10.1126/science.abq0516
Thank you for your interesting post about our molecule.
Our understanding of the origin of solid state interaction of octafluorocubane was improved.
Regarding to this post, I would appreciate your opinion on the following matter if you could share it with me.
The thermochemistry of cubane (C8H8) has already been investigated well (e.g. J. Phys. Chem. A 2015 119 2998, Org. Process. Rev. Dev. 2013, 17, 1503). And its boiling point was reported to be 161.6 degC. It is comparable to octafluorocubane (C8F8). We think it is also quite interesting but we don’t have any explanation on this similarity. How do you think the origin of high boiling point of C8H8?
Dear Masa,
Thanks for the comments. Again our crystallographer Andrew was asked. Thanks Andrew!! He tells me “there are actually only two unique carbon atoms (C1 and C2) and two unique associated hydrogen atoms.”
“H1 makes 9 contacts of ca. 2.7 Å (to another H), but 3 of those are to the parent cubane, so the intermolecular count is 6, 4 of which are to copies of H1, and 2 to copies of H2. Similarly, H2 makes 9 contacts of ca. 2.7 Å, but 3 of those are to the parent cubane, so the intermolecular count is also 6, all to copies of H1.”
So each hydrogen is involved in six contacts of ca. 2.7 Å to another H. Although each is weak (<0.5 kcal/mol ?) the accumulation adds up to a boiling point of 162C!