Derek Lowe’s blog has a recent post entitled A Downside to Oxetane Acids which picks up on a recent article[1] describing how these acids are unexpectedly unstable, isomerising to a lactone at a significant rate without the apparent need for any catalyst. This is important because these types of compound occur frequently in the medicinal chemistry literature.
The isomerism is reported to occur quite slowly but significantly in the pure substance, being complete in around a year. Any uncatalysed mechanism must comprise a proton transfer to the oxygen of the oxetane followed by an Sn2 displacement at the methylene group in the protonated oxetane. This could be stepwise or concerted (the latter shown with arrows above). To determine the answer, an ωB97XD/Def2-TZVPP/SCRF=chloroform calculation was performed (FAIR data DOI: 10.14469/hpc/10820) which clearly shows a concerted reaction, albeit one in which a proton transfer (IRC ~-1.5) preceeds the Sn2 displacement with retention of configuration! The transition state appears to have no biradical character. The activation free energy is ΔG‡ 49.9 kcal/mol and the reaction is clearly exoenergic.
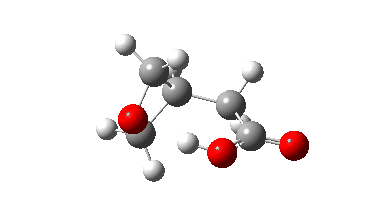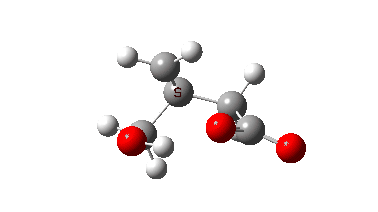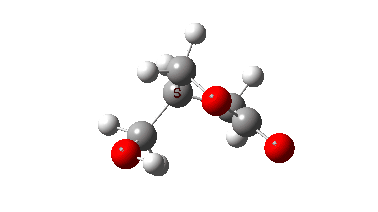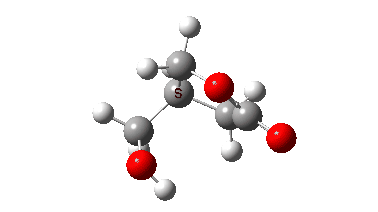
The dipole moment response shows that the proton transfer induces a larger dipole moment, which then reduces as the Sn2 reaction occurs.
The calculated free energy barrier of ~50 kcal/mol is ~15-20 kcal/mol too high to occur thermally and so the observed reaction either occurs via a different mechanism, perhaps bimolecular in which one molecule of the oxetane-carboxylic acid acts as a catalyst for a second molecule to rearrange, or one in which a stronger external acid catalyst operates, such as traces of other acid in the system. Exploring the sensitivity to substituents on the oxetane ring (CH3, CF3, etc) might also cast light on the mechanism, as might testing the stereochemistry of the two carbons next to the oxygen of the oxetane; do they retain, invert or scramble the configuration at these two centres?
DOI: 10.14469/hpc/10863 and 10.14469/hpc/10862
References
- B. Chalyk, A. Grynyova, K. Filimonova, T.V. Rudenko, D. Dibchak, and P.K. Mykhailiuk, "Unexpected Isomerization of Oxetane-Carboxylic Acids", Organic Letters, vol. 24, pp. 4722-4728, 2022. https://doi.org/10.1021/acs.orglett.2c01402
Most of the compounds studied in [1] are solids at room temperature, so intermolecular hydrogen bonding could be catalytic? Similarly, the OH group in the product could catalyse neighbouring molecules?
Indeed so Mike, as I wrote “bimolecular in which one molecule of the oxetane-carboxylic acid acts as a catalyst for a second molecule to rearrange”
Hello Prof. Rzepa,
In this post, RMS gradient norm showed a dip at IRC ~-1.5 which suggest that proton transfer is asynchronous.
I have a question regarding this, can we say that there can be a hidden intermediate present on the potential energy surface of this reaction?
Yes indeed, my comment “a proton transfer (IRC ~-1.5) preceeds the Sn2 displacement with retention of configuration” implies a hidden, in this case zwitterionic intermediate with a large dipole moment.