When you talk π-aromaticity, benzene is the first molecule that springs to mind. But there are smaller molecules that can carry this property; cyclopropenylidene (five atoms) is the smallest in terms of atom count I could think of until now, apart that is from H3+ which is the smallest possible molecule that carries σ-aromaticity. So here I have found what I think is an even smaller aromatic molecule containing only four carbon atoms. And it is not only π-aromatic but σ-aromatic.
Let me go through the analysis (using a CCSD(T)/Def2-TZVPPD calculation, DOI: 10.14469/hpc/10226).
- Four carbons contain 16 valence electrons for bonding.
- Eight of these are conventional, forming four C-C single bonds around the 4-ring.
- Eight are left over, and these partition into a set of six and a set of two.
- The set of two are in p-π atomic orbitals and form a 4n+2 (n=0) aromatic system
- The set of six are in σ-sp AOs and form a 4n+2 (n=1) aromatic system.
- The three σ-MOs all contribute to the central C-C bond, particularly σ3 and σ2 in different ways.
- σ2 also reminds of [1.1.1]-propellane, where the two σ-electrons are in effect external to the central C-C bond, but spin coupled to form what might be called a σ exo-bond. There is also similarity to the exo bond in C2.
- The dissociation energy of the central bond can be estimated at 28 kcal/mol from the triplet state energy.
| Bonding MOs for C4. Click image to load 3D model |
|
|---|---|
| π1 | |
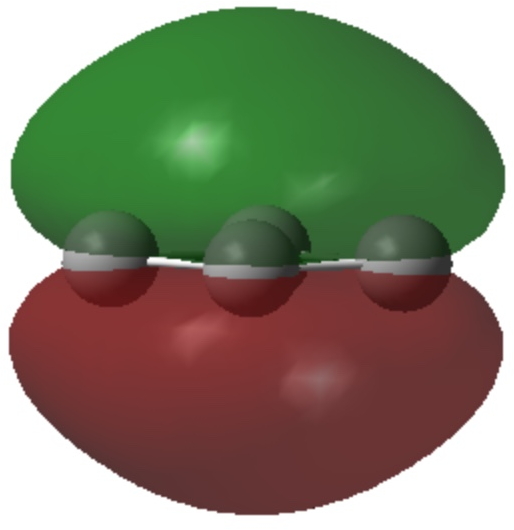 |
|
| σ3 | σ2 |
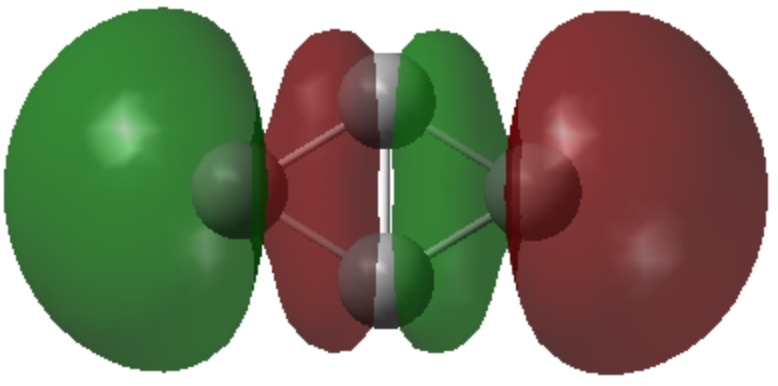 |
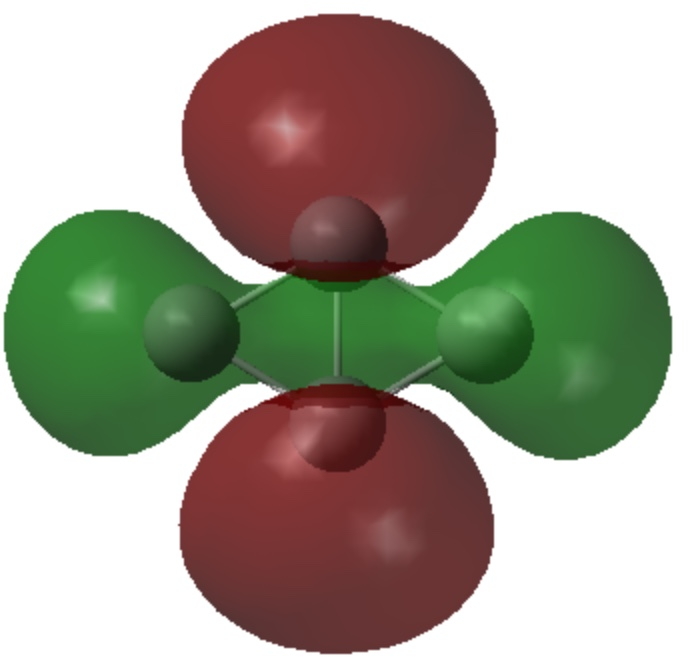 |
| σ1 | |
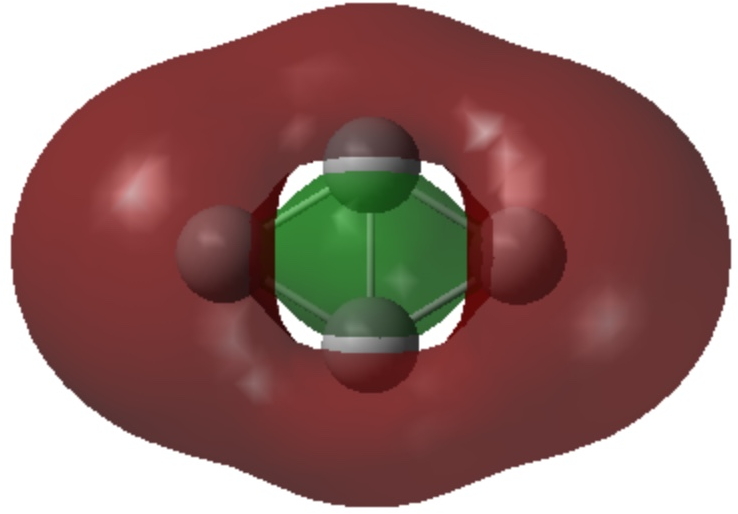 |
|
So this little molecule carries a lot of diversity in its chemical bonding; an ideal candidate perhaps for a tutorial in bonding theory of organic molecules?
The post has DOI: 10.14469/hpc/10252
Would it be lower energy folded into a tetrahedron? Then there would be six sigma bonds. Would the angular strain energy be too large?
The form of C4 with Td symmetry has two electrons in a triply degenerate Td orbital and so must undergo Jahn-Teller distorsion to the lower C2v symmetry. This species is 106.0 kcal/mol higher in free energy than the bicyclic aromatic form and is a second order transition state. The first of these -ve force constants has vectors that distort back to the bicyclic form.
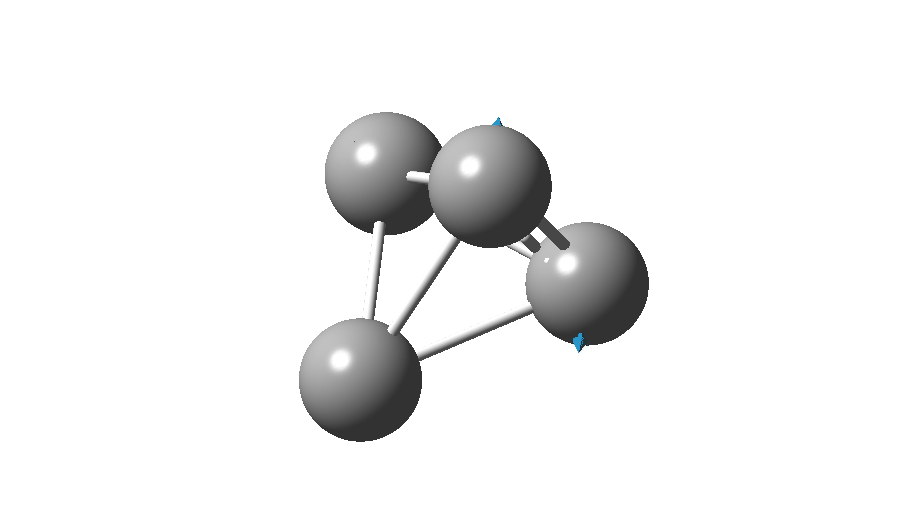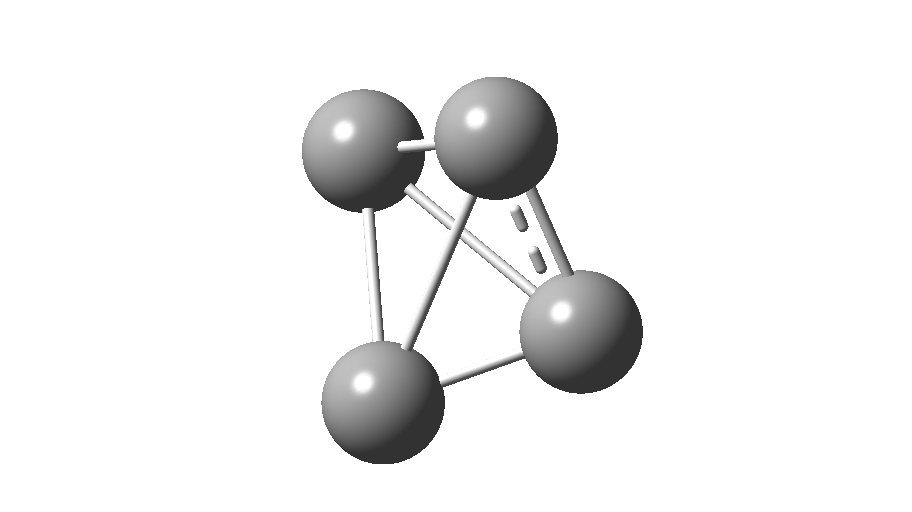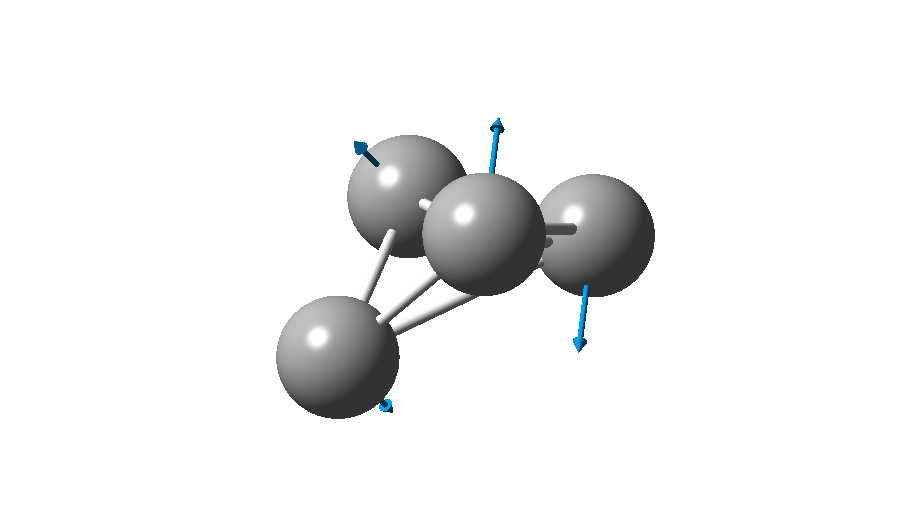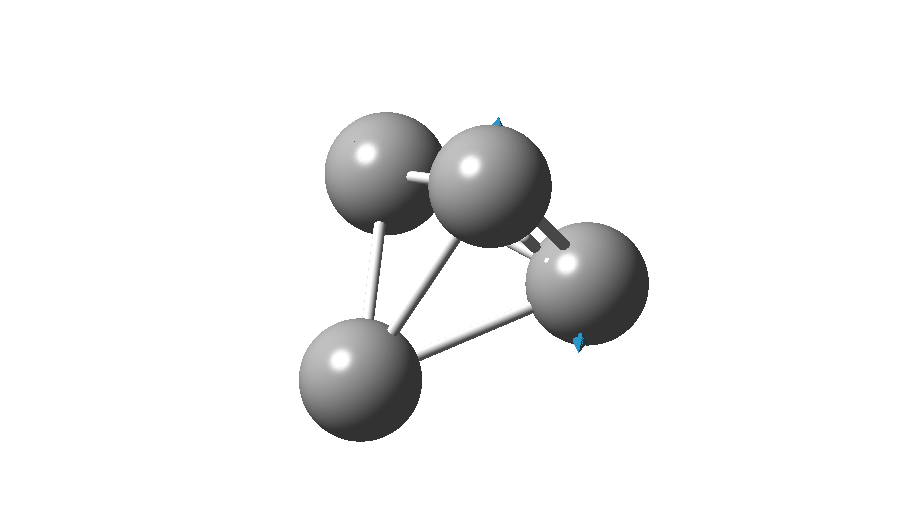
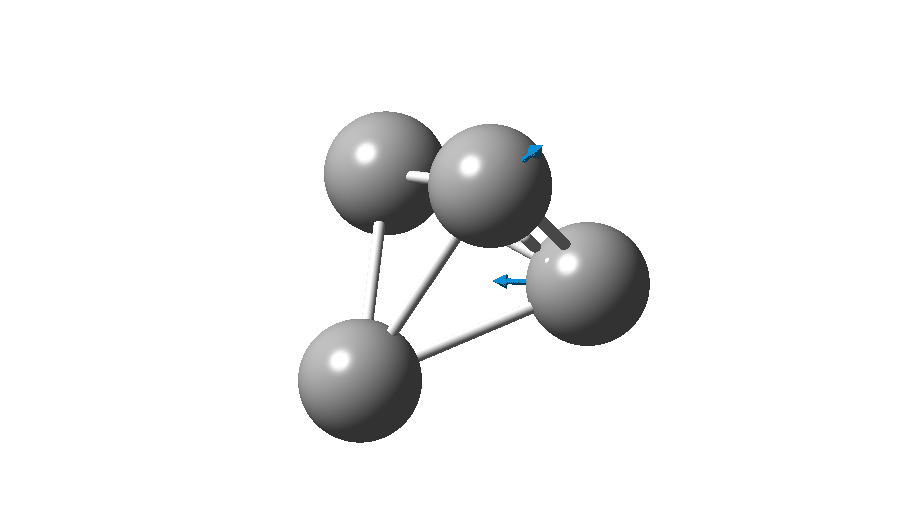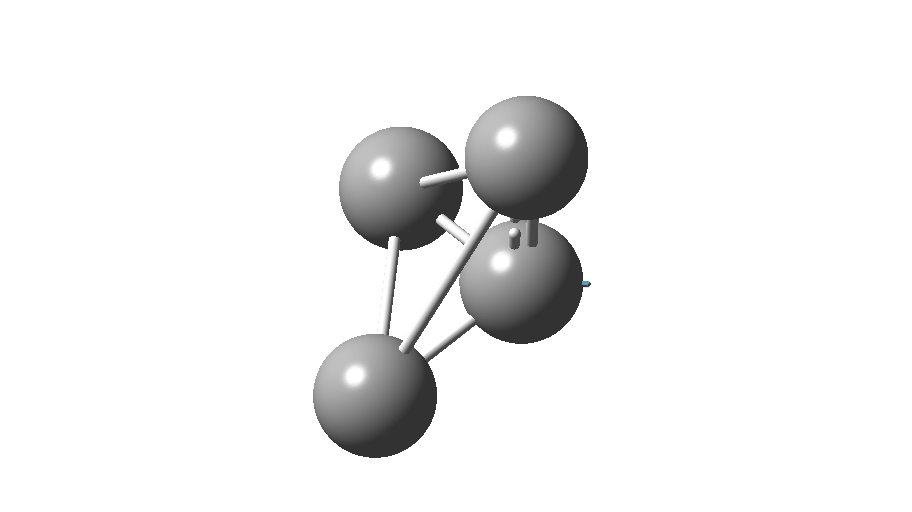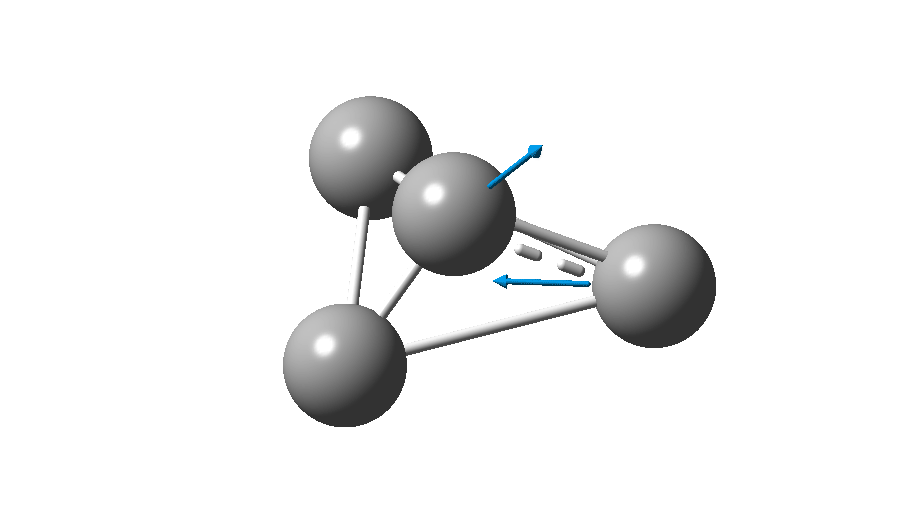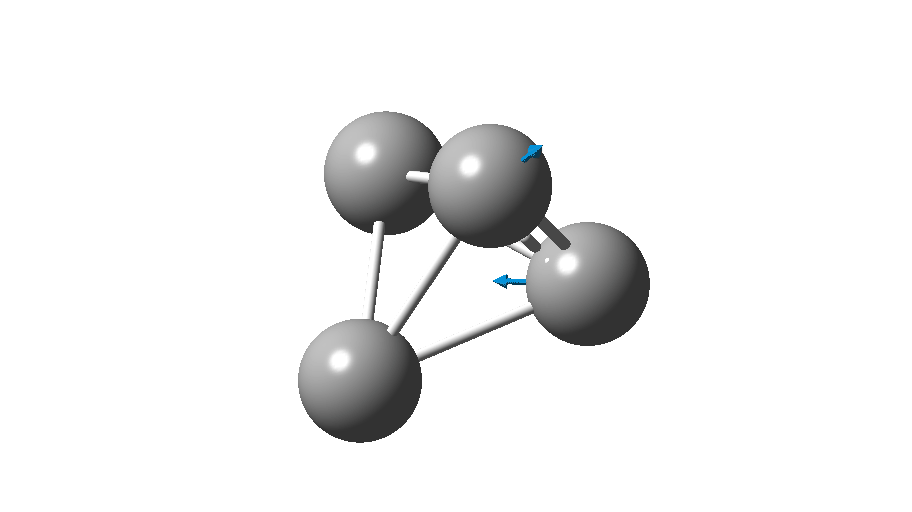
The second has vectors that distort to a “methylenecyclopropene” like structure (but without the hydrogens). So the answer to your question is that the tetrahedral form is not stable.
That’s a very neat electronic structure – does it make this the most stable isomer of C4?
Not comprehensively investigated, but it is certainly more stable than linear C=C=C=C by ΔG 8.4 kcal/mol at the CCSD(T)/Def2-TZVPPD level (see DOI https://doi.org/10.14469/hpc/10226).
I show here the calculated IR spectrum.
Who knows, it might exist in inter-stellar space!