Carbon dioxide is much in the news, not least because its atmospheric concentration is on the increase. How to sequester it and save the planet is a hot topic. Here I ponder its solid state structure, as a hint to its possible reactivity, and hence perhaps for clues as to how it might be captured. The structure was determined (DOI 10.1103/PhysRevB.65.104103) as shown below.
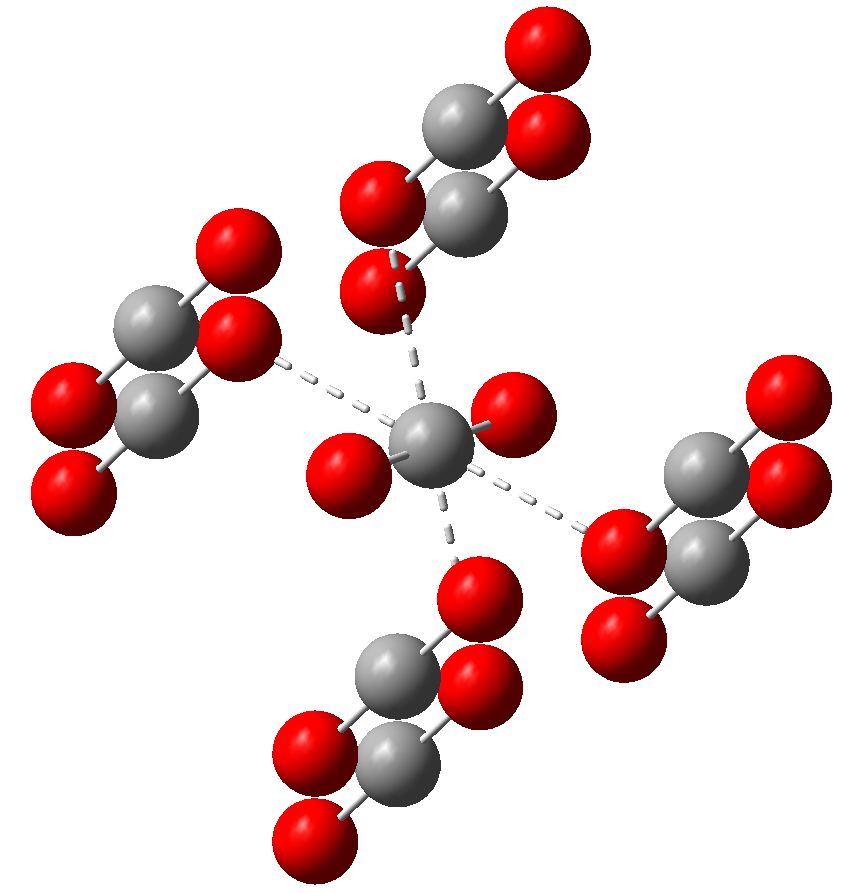
The structure of solid carbon dioxide. Click for 3D

Designed hexacoordinate carbon. Click for 3D
If oxygen atoms can approach the carbon in CO2 to within ~2.4Å, an interesting question can be posed. How close can another carbon get to CO2 without actually reacting and forming a new molecule? C-C bonds, even weak ones, are so much more interesting than C-O bonds! It would have to be a particularly nucleophilic carbon, of course. A search of the August 2010 version of the Cambridge structural database (CSD) reveals no really close approaches of another carbon to CO2. Only about 8 weak examples are found, and here the C-C distances are ~3.0-3.2Å, with the O=C=O angle in the CO2 never less than 170°. In this context, there is an intriguing and very recent report (which has not yet made it into the searchable CSD) of the structure of CO2 trapped in a cavity next to what was claimed to be a molecule of 1,3-dimethyl cyclobutadiene, or CBD (see 10.1126/science.1188002 and the discussion of this article in my earlier blog post). The focus in that report was on the “Mona Lisa of organic chemistry”, namely the CBD unit. One feels that the structure of the adjacent CO2 was of lesser interest to the authors. According to a visual image of this system, the CBD and CO2 pair show quite an intimate approach via their carbon atoms (a ghostly C-C bond is clearly represented). This raises the interesting question of whether the description of this pair should be of two intimate but nevertheless separate and relatively unperturbed molecules not connected by a covalent bond (“more indicative of a strong van der Waals contact than of covalent bonding“) or of a pair fully bound by a covalent C-C bond between them?
The issue of what is an interaction, and what is a bond continues to raise its often controversial head. And quantum theory continues to provide a multitude of interpretations as well.
Tags: 13-dimethylcyclobutadiene, Borboiu, Cambridge, carbon dioxide, CBD and CO, CCDC, CDS, crystal structure, crystalline calixarene network, guest, host, Hypervalency, Interesting chemistry, often controversial head, van der Lee
[…] Henry Rzepa Chemistry with a twist « Solid carbon dioxide: hexacoordinate carbon? […]
[…] van der Waals radii, and very typical of oxygen…electrophilic carbon interactions (see discussion here for more details). We can reasonably assume its real. It is supported by a small NBO perturbation […]
[…] Fortunately, crystal structures are available. Let me start with n-butyl lithium, a very commonly used reagent[1]. This forms a complex cluster of six lithiums, in which each metal is surrounded by three CH2- terminii of the n-butyl anion, and vice-versa, each CH2- group is in contact with three lithium atoms (making the carbanionic carbon in effect hexa-coordinate). […]
I note here two recent publications (doi: 10.1073/pnas.1118791109 and 10.1103/PhysRevLett.108.125701) where the structure of carbon dioxide at very high pressures has been solved (CO2-V). It contains tetrahedral CO4 units with no remaining C=O double bonds.
[…] It is worth exploring the immediate environment of each of these types. The monocarbon is in fact hexa-coordinated by two Os and four La, a form of carbon coordination that was only relatively recently […]