Occasionally, someone comments about an old post here, asking a question. Such was the case here, when a question about the dipole moment of cyclopropenylidene arose. It turned out to be 3.5D, but this question sparked a thought about the related molecule below.
Of the two resonance forms show above, the one on the left is a zwitterion resulting in the formation of an aromatic cyclopropenium ring, with the charge balanced by the acetylide anion.‡ The calculated structure (ωB97XD/Def2-TZVPP/SCRF=chloroform, Data DOI 10.14469/hpc/8399 ) shows a short terminal CC bond which indicates a significant degree of delocalisation of the three membered ring.
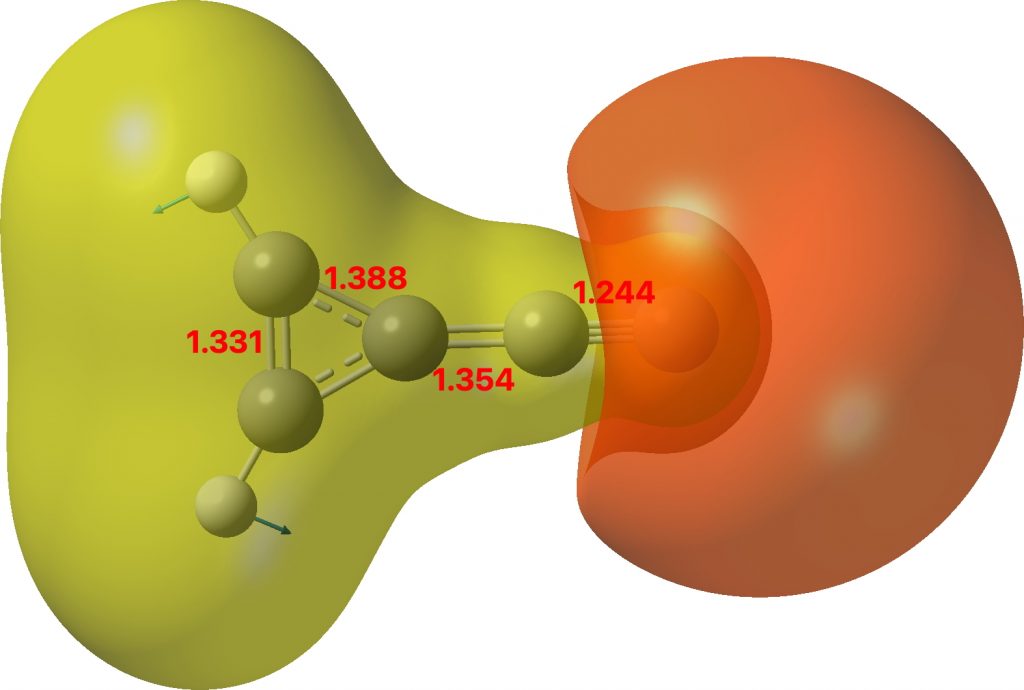
Molecular electrostatic potential
The molecular electrostatic potential (above) agrees with a large dipole moment of 11.9D. This is certainly up there with the molecule suggested in 2016 as being the most polar molecule neutral compound synthesised[1] and is a fair bit smaller than that candidate. A brief search of the literature (Scifinder) suggest that the molecule is currently unknown. Anybody fancy making it?
The Data DOI by the way gives you access to the other outputs from the calculation, which would include the molecular orbitals and the molecular vibrations which I have not shown here.
‡ Such zwitterions are known as unstable intermediates. See e.g. [2] for examples.
References
- J. Wudarczyk, G. Papamokos, V. Margaritis, D. Schollmeyer, F. Hinkel, M. Baumgarten, G. Floudas, and K. Müllen, "Hexasubstituted Benzenes with Ultrastrong Dipole Moments", Angewandte Chemie International Edition, vol. 55, pp. 3220-3223, 2016. https://doi.org/10.1002/anie.201508249
- H.S. Rzepa, "Routes involving no free C <sub>2</sub> in a DFT-computed mechanistic model for the reported room-temperature chemical synthesis of C <sub>2</sub>", Physical Chemistry Chemical Physics, vol. 23, pp. 12630-12636, 2021. https://doi.org/10.1039/d1cp02056k
So the next question would be, will Cyclopropenylidene react with C2 to make that acetylide?
Good question. To answer, I must first comment on how C2 itself might be generated. An apparent route to this species via the unimolecular fragmentation of the intermediate ylid –CC-I+Ph was originally suggested as a mild chemical (rather than high temperature thermal) synthesis (DOI: https://doi.org/10.1038/s41467-020-16025-x). It appears that rather than this unimolecular fragmentation, the ylid itself reacts with another molecule via a bimolecular mechanism in which I-Ph is displaced (DOI: https://doi.org/10.1039/D1CP02056K)
So the question above can now be recast as: will Cyclopropenylidene react with –CC-I+Ph to form the desired acetylide. I will calculate the transition state for this process and report back.
The reaction between cyclopropenylidene and –CC-I+Ph has the expected small barrier of ΔG 15.5 kcal/mol. Mind you the reaction between –CC-I+Ph and itself has a similar barrier. Then there is the problem of generating cyclopropenylidene itself. Thus the reaction between two transient species is unlikely and each is liable to react not only with the other but also with itself.
As for cyclopropenylidene, it might be generated by photolysis or thermolysis of 1,2-diazaspiro[2.2]penta-1,4-diene. This species however has never been reported.
Both dicarbon and cyclopropenylidene have been reported in molecular gas clouds by astrophysicists. Dicarbon goes back (at least) to
September 1983 The Astrophysical Journal 271(2)
DOI:10.1086/184102
and cyclopropenylidene to
June 1989 The Astronomical Journal 97(5):1403-22
DOI:10.1086/115081
So is there a realistic prospect of these species occurring together, and reacting sufficiently fast, over astronomical time, to form the acetylide studied here?
No realistic prospect. The density of such molecules in outer space is about one per cubic metre. The probability of a collision is essentially zero.
Whilst your comment is correct for the bulk of interstellar space, carbon is synthesised as single atoms and somehow gets to form something over 200 molecules according to Wikipedia:
https://en.wikipedia.org/wiki/List_of_interstellar_and_circumstellar_molecules
The list includes dicarbon and cyclopropenylidene and runs up to species like benzonitrile and cyanopentaacetylene which require substantial chemistry to make them from individual atoms. No doubt the atmospheres of red giant stars are a major factory as there’s abundant first row elements, a temperature low enough for chemistry, and enough black stuff to keep the UV down to levels where organic molecules can survive. As this is blown out into interstellar space the concentration and temperature fall which might allow synthetic chemistry to continue for some time. Someone must have a computer model of these processes and some idea how the mixture evolves.