In the preceding post, I looked at a computed mechanism for the hydrolysis of a ketal by water. Of course, pure water consists of three potential catalysts, water itself or [H2O], and the products of autoionisation, [OH–] and [H3O+]. The latter are in much smaller concentration, equivalent to a penalty of ~11.9 kcal/mol on any free energy barrier. Here I take a look at these ion-catalysed routes to see if that penalty can be overcome.
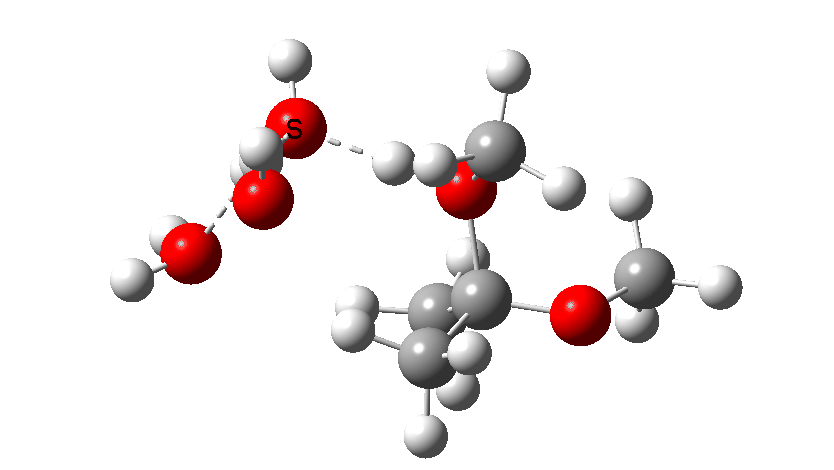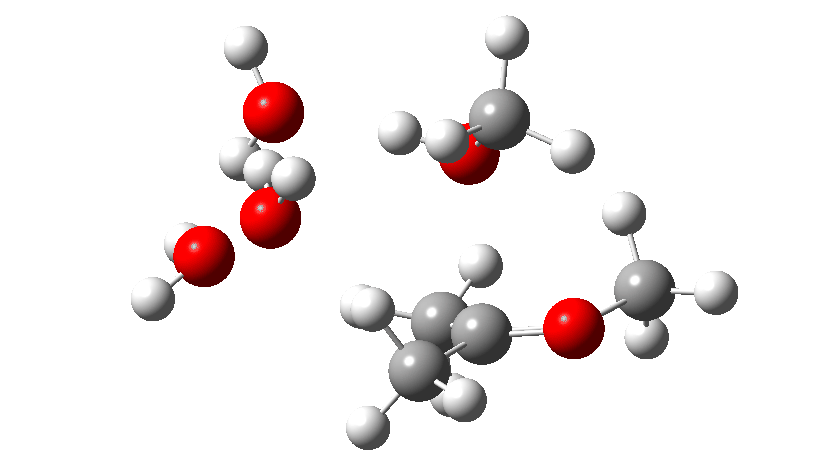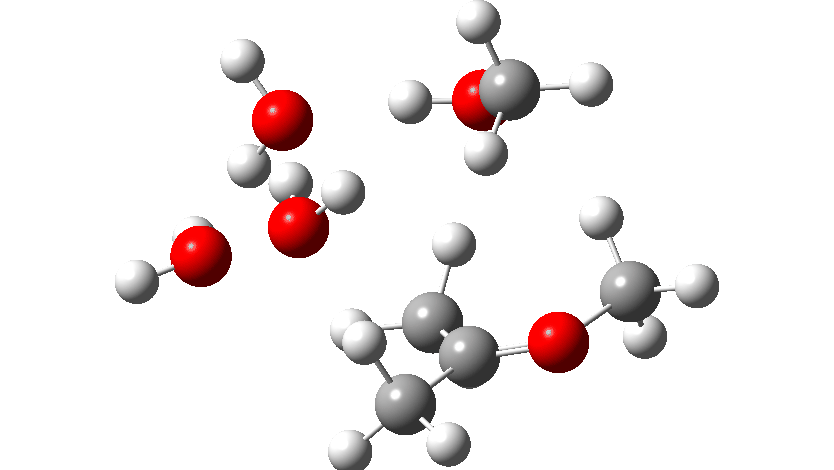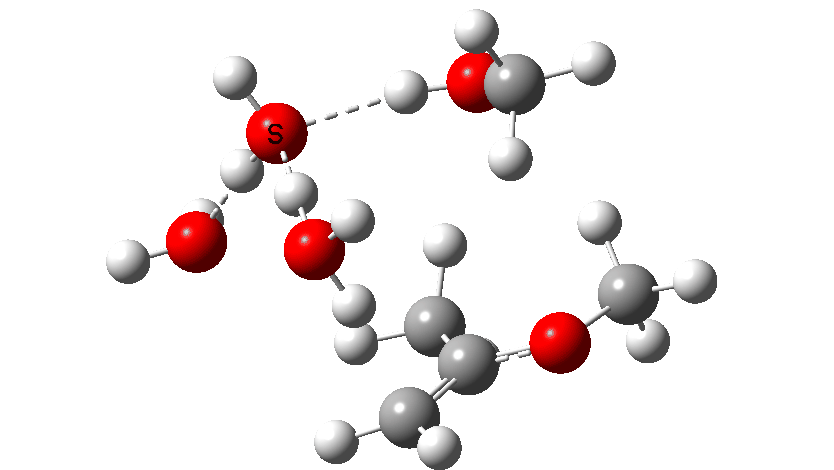
The calculations can be found at FAIR DOI: 10.14469/hpc/8071. The hydroxyl anion route is shown below, and has a computed free energy barrier of 34.1 kcal/mol. Only the first TS is shown here, since already we know that the barrier must be at least that high regardless of subsequent steps. This means that hydroxide anion catalysis must be insignificant.
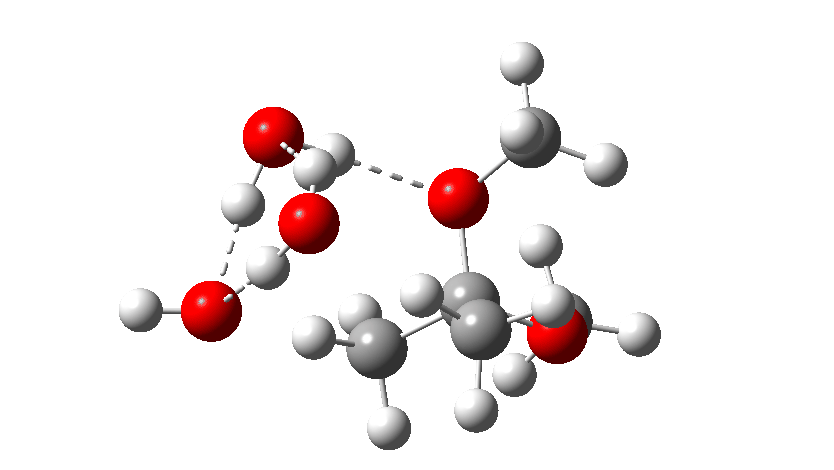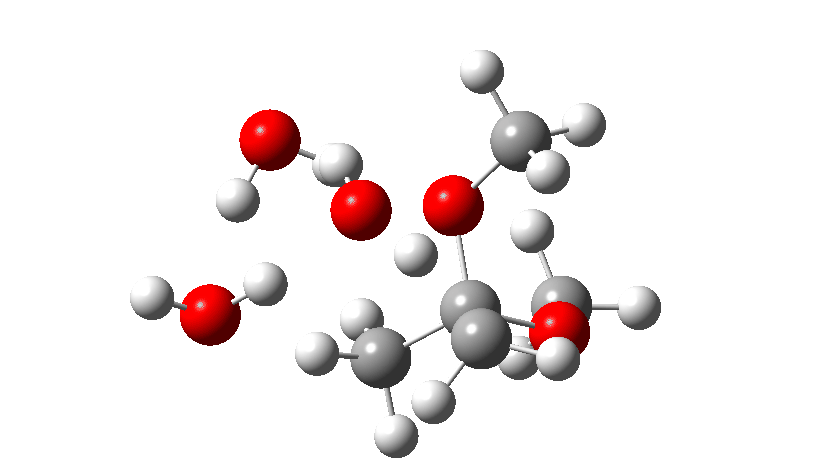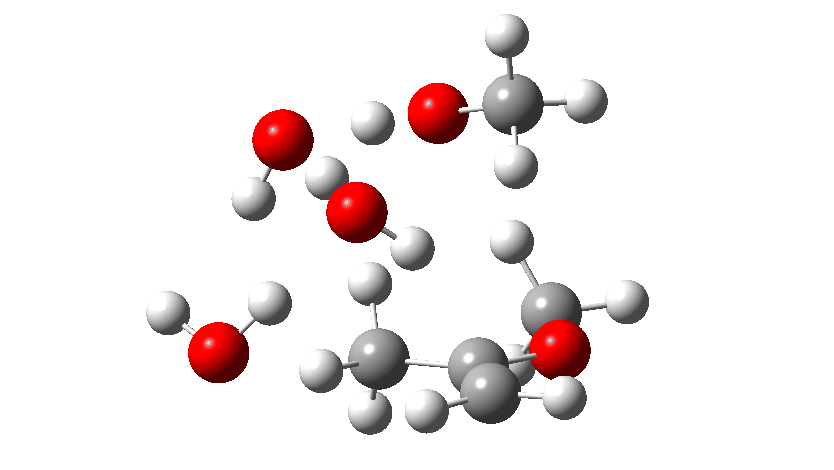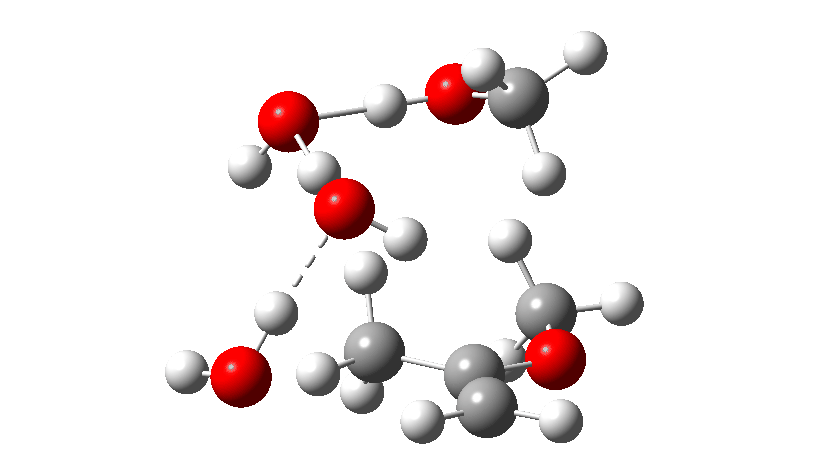
Next, the acid catalysed route, which is a two-stage path.
Firstly one methoxy group is protonated from the hydronium cation and the C-O bond cleaves (IRC 14 to 8). In the second stage, a water molecule abstracts the C-H hydrogen, regenerating the hydronium cation (IRC 2 to -2), during which process the transition state occurs to form an enol. This transition state has a free energy barrier of 16.2 kcal/mol. Both the second TS (13.4 kcal/mol) which reverses this to form a hemiacetal and TS3, which eliminates the second methanol to form a ketone (-3.5 kcal/mol) are lower in energy.
So we conclude that the hydronium cation catalysed route is easily accessible at ambient temperatures. Adding ~11.9 kcal/mol to account for the [H3O+]-7 concentration of this ion in water gives a “pure water” barrier of ~28 kcal/mol, which corresponds to a rather slow but viable hydrolysis (ie ~days half life) at ambient temperatures. Armed with this information, we can now start to address the hydrolysis of a bio-active ketal-based herbicide in water.