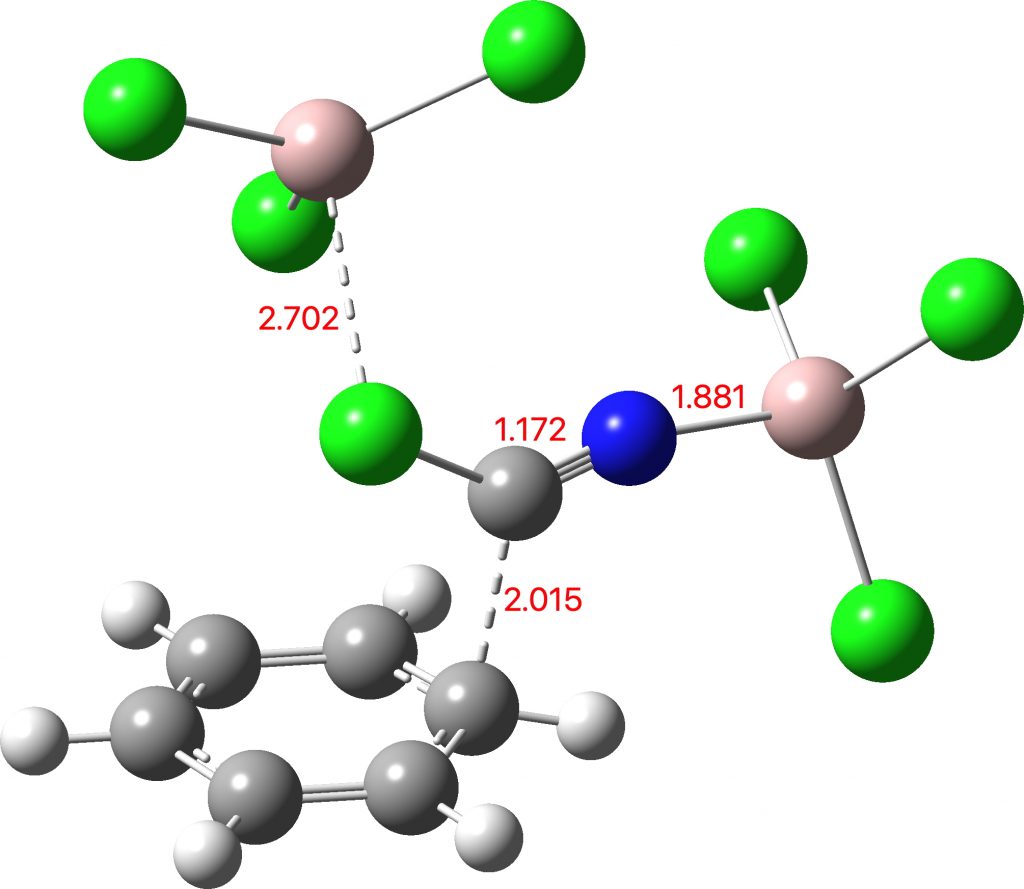
I asked the question in my previous post. A computational mechanism revealed that AlCl3 or its dimer Al2Cl6 could catalyse a concerted 1,1-substitution reaction at the carbon of Cl-C≡N, with benzene displacing chloride which is in turn captured by the Al. Unfortunately the calculated barrier for this simple process was too high for a reaction apparently occuring at ~room temperatures. Comments on the post suggested using either a second AlCl3 or a proton to activate the carbon of the C≡N group by coordination on to nitrogen. A second suggestion was to involve di-cationic electrophiles. Here I report the result of implementing the N-coordinated model below.
Click on image for 3D model
The free energy barrier ΔG‡298 is 20.8 kcal/mol (FAIR Data DOI: 10.14469/hpc/7584), which corresponds to a facile reaction at room temperatures. There does not seem to be any need to invoke super-reactive di-cationic electrophiles in this instance. This is yet another illustration that computational modelling nowadays is good enough to flag unviable mechanisms, and hence to instigate a search for a better model.
Is a two-step addition-elimination mechanism involving ClCN coordinated to one AlCl3 via nitrogen possible? As a corollary, which is preferred, bonding to AlCl3 via Cl or via N?
A related question – has the mechanism by which the well-studied acyl chloride-AlCl3 complexes (O-Al bond) give acylium ions with loss of Cl4Al- been studied? That step is glossed over in teaching the Friedel-Crafts acylaion mechanism.
I once checked a few references starting from “March’s Advanced Organic Chemistry”, and one (from 1996) I found interesting is https://doi.org/10.1021/ja9624331 and (more recent) https://doi.org/10.1039/C3OB27094G
Both represent experimental studies with kinetics by spectroscopy. The introduction to the articles, and the respective chapter in the March book (11-17) are worth reading regarding your question.
All kinds of variations with electrophiles including free acylium cation (as SbF6 salt), O-complexed acid chlorides, Cl-complexed acid chloride, O,Cl-bis-complexed acid chloride have been considered and may be operative under specific conditions.
Maybe even better as authoritative review: A. Molnar, G. A. Olah, G. K. S. Prakash, Chapter 8 (“Acylation”) in “Hydrocarbon Chemistry”, 3d ed 2018, J. Wiley and Sons. https://doi.org/10.1002/9781119390541.ch8
Here is an energy profile in which cyanogen chloride is replaced by acetyl chloride, one of the examples noted above. The barrier corresponds to a very facile thermal reaction. The mechanism is as with cyanogen chloride, in that the departure of the chloride from the acyl group to become AlCl4– is synchronous with the formation of the benzene C-C bond to the acyl group. Clearly such a synchronous route is very viable. To exclude them, other mechanisms would have to be computed and compared in energy.
The mechanism shown above with ClCN coordinated to one AlCl3 is significantly lower in energy than the one where AlCl3 is coordinated to another AlCl3.