Here I investigate a recent report[1] of a new generation of polyesters with the intrinsic properties of high crystallinity and chemical recyclability. The latter point is key, since many current plastics cannot be easily recycled to a form which can be used to regenerate the original polymer with high yield. Here I show some aspects of this fascinating new type of polymer.
The starting monomer is 2-thiabicyclo[2.2.1]heptan-3-one, which is easily prepared on a 50g scale. When polymerised with the organocatalyst IMES (below) it produces a stereoregular threodisyndiotactic polymer of the structure shown above. Various other catalysts produced different stereochemistries.
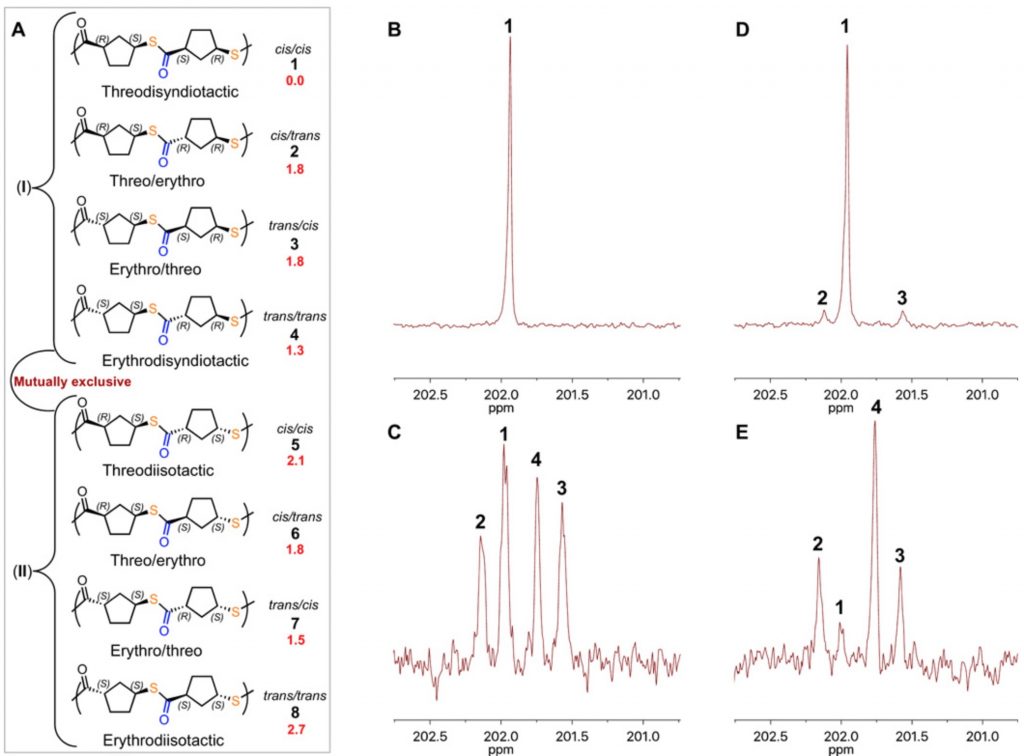
The 13C NMR spectrum of the IMES-catalysed polymer showed just a single 13C carbonyl resonance, and the stereochemical assignment was based in part on DFT geometry optimisation calculations and then obtaining relative free energies of a model dimeric molecule, capped as a methyl ester rather than a benzyl ester. These showed that the (R,S,S,R) stereoisomer was lower than the next lowest by ~1.3 kcal/mol (Figure below). These calculations were done using a DFT procedure (BP86) that does not include dispersion corrections and so I could not but help wonder whether the conformational analysis was entirely reliable, especially if it is to be used to “tentatively” assign stereochemistry (the authors’ own description, Figure 3 below) to the pure stereopolymer B.
I thought I might make my own very quick investigation of the conformation of these quite flexible monomers. I proceeded as follows:
- I drew the molecule as a tetramer as shown above, for the (R,S,S,R) stereoisomer and saved the coordinates as a molfile. This adds approximate three dimensionality based on the hashes and wedges. I used a tetramer to give it sufficient size to coil around on itself if it wanted to; a dimer is a little too small to do this.
- This is then fed to a program which can refine such approximate structures using molecular mechanics (the MMFF94s force field, which intrinsically includes attractive dispersion terms). In this instance, I used Avogadro, producing a more accurate 3D model of the system.
- This was then subjected to semi-systematic conformer searching using Avogadro. The lowest energy of 27 conformations found was then taken forward for optimisation using quantum mechanical procedures.
- The first of these used PM7, a rapid semi-empirical procedure that includes the 3rd generation Grimme dispersion correction.
- This was then subjected to B3LYP+GD3BJ/Def2-SVP final refinement (again a procedure that includes a modified GD3 dispersion term with BJ damping).‡
The original dimer model reported in the supporting information for the article as stereoisomer 1 is shown below next to the tetramer optimised using the procedure described above.
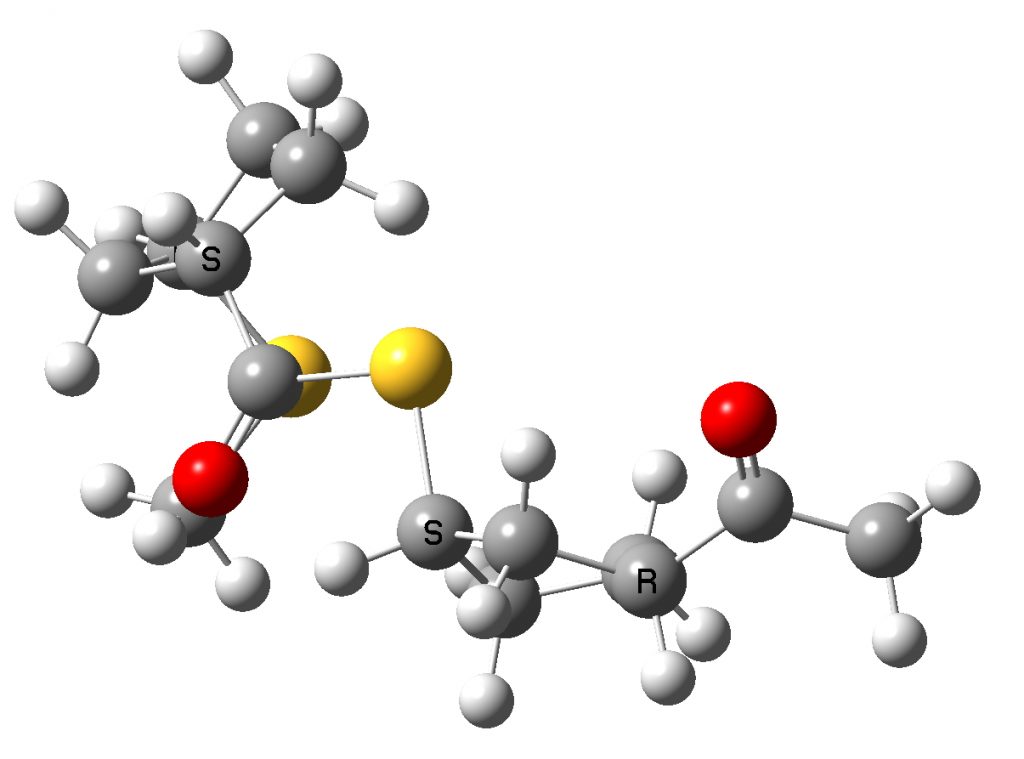
Conformation of literature stereoisomer 1, (R,S,S,R). Click to show 3D rotatable mode
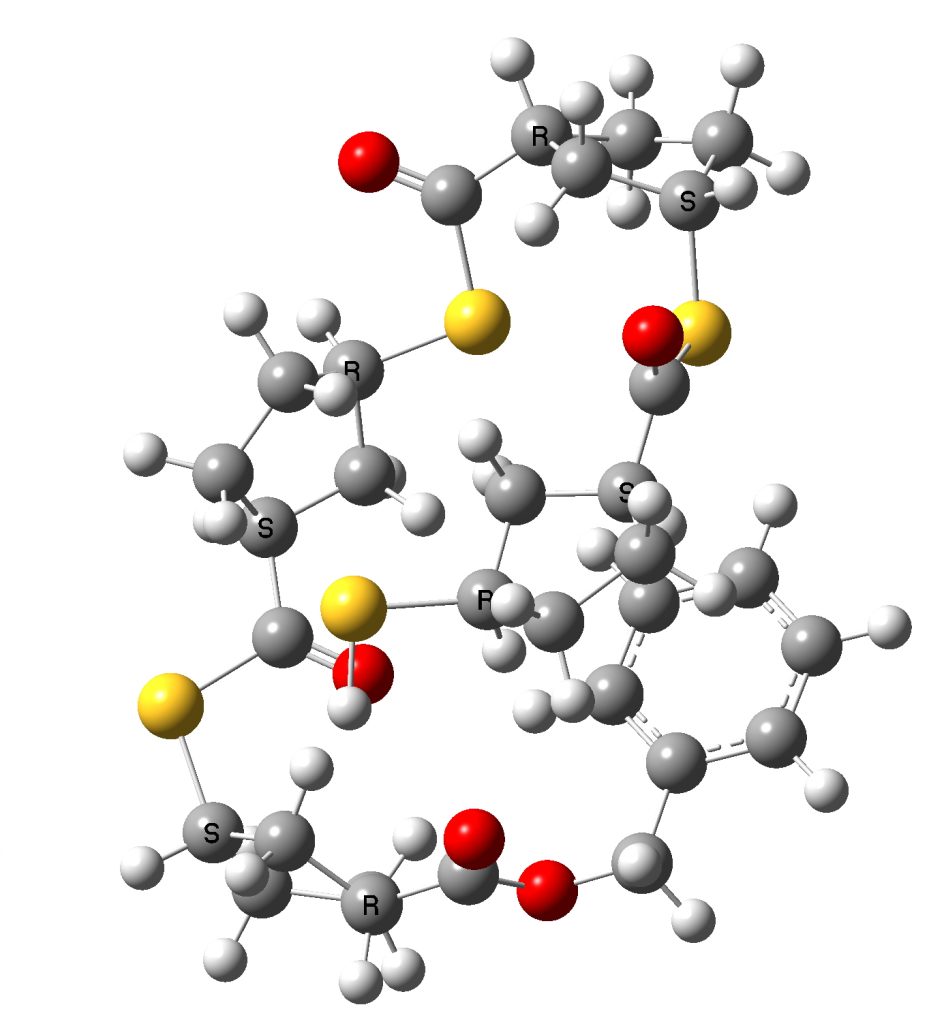
Conformation of 1 as obtained by the procedures above on the tetramer. Click to show 3D rotatable model.
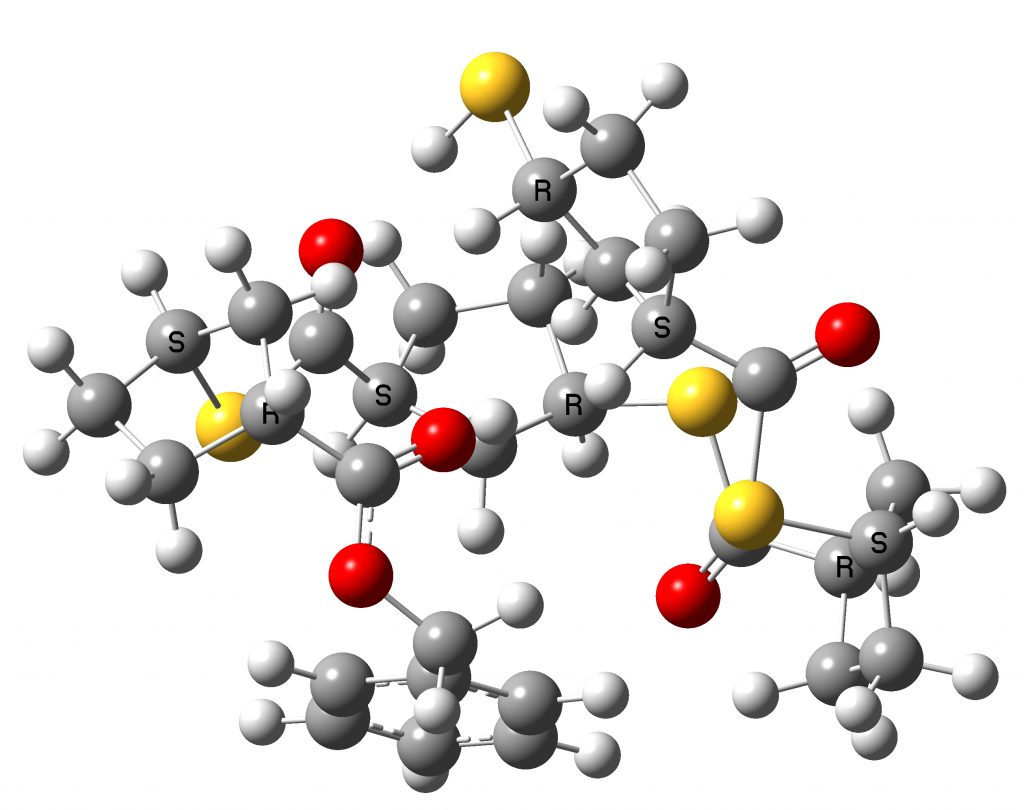
Conformer 5.1 kcal/mol lower in free energy
You can explore both conformations yourself by clicking on the images above to get a 3D rotatable model. They do look rather different, in that the tetramer is wound back upon itself (a sort of hairpin looping), encouraged by the face of the phenyl group which accepts a 5-ring by dispersion attractions. The tentative assignment[1] that the (R,S,S,R) stereochemistry corresponds to that of the pure polymer B produced using the IMES catalyst must probably be just that, tentative. No doubt crystallography will verify this in due course.
We see here a possible glimpse of the future of plastics, whereby highly recyclable polymers can be produced that recover the original monomer with very little loss. All that is needed now is that the plastic is efficiently recycled and not just dumped into the oceans!
‡ Data at DOI: 10.14469/hpc/7375
References
- C. Shi, M.L. McGraw, Z. Li, L. Cavallo, L. Falivene, and E.Y. Chen, "High-performance pan-tactic polythioesters with intrinsic crystallinity and chemical recyclability", Science Advances, vol. 6, 2020. https://doi.org/10.1126/sciadv.abc0495