Text books often show the following diagram, famously consolidated over many years by Emil Fischer from 1891 onwards. At the top sits D-(+)-glyceraldehyde, to which all the monosaccharides below are connected by painstaking chemical transformations.
In this notation, D (for all these structures) indicates the absolute configuration of the series, deriving from placing the last highest priority group before an achiral carbon at the bottom of the representation (the hydroxyl group) to the right of the diagram (= dextro = D). The mirror images of all these species would be designated L (since the hydroxide group would now be on the left). The challenge emerges in connecting this absolute configuration to some measurable property, and during the late 19th century, the only feasible measurement would be the sign of the optical rotation. It was decided that since no method existed at the time to connect absolute configuration to the sign of optical rotation, D-glyceraldehyde (nowadays more commonly referred to by its CIP notatation (R)-glyceraldehyde), would be by convention connected to the enantiomer giving a positive rotation, hence D-(+)-glyceraldehyde. This species has indeed mostly been reported as having a small positive rotation of e.g. +9.2[1] in water. If a future procedure were to confirm that the D-enantiomer had a positive rotation, Fischer’s guess would be proven correct. Possibly.
I quickly mention that this experiment was of course carried out in 1951, as noted in the previous post[2], but on a rubidium tartrate salt and not on glyceraldehyde itself (which is a liquid). Since the two could be connected by chemical synthesis, Fischer’s guess was indeed confirmed as correct. This proof rapidly made D-(+)-glyceraldehyde less relevant as the tentpole of absolute configuration (in sugars) and people stopped worrying about it. But the assertion that the D/(R) enantiomer has a positive rotation by indicating (+) continues in all the diagrams related to the above that I have come across. So is it correct?
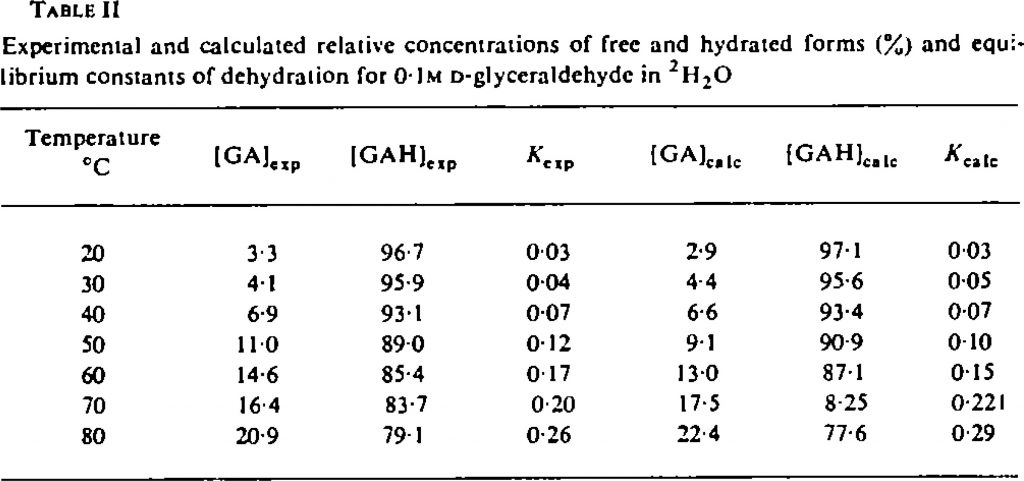
Well, an earlier attempt in 1937 to assign optical rotation to absolute configuration had been attempted by Kirkwood[3] using quantum mechanical theory to make that connection. I thought I might apply the modern version of that approach to (R)-glyceraldehyde, using the same procedure I had tried earlier. A conformational analysis of the molecule, followed by dispersion-corrected calculation of free energies and then of the optical rotations gives the following table. Data is at 10.14469/hpc/6436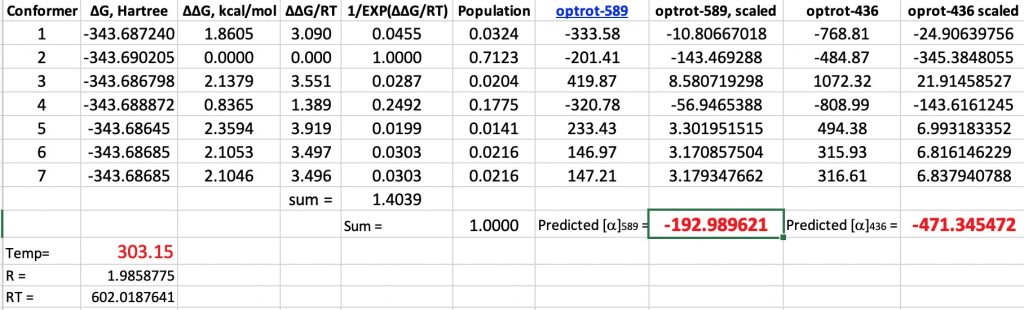 These calculations show that the rotation is strongly negative! Can they be in serious error? Perhaps however there are two different species in solution. Indeed, the equilibrium below is shown to favour the rhs for aldehydes, as shown by the following concentration profile as a function of temperature.[4] This shows that at 30°, only 4% of this molecule is in the aldehyde form.
These calculations show that the rotation is strongly negative! Can they be in serious error? Perhaps however there are two different species in solution. Indeed, the equilibrium below is shown to favour the rhs for aldehydes, as shown by the following concentration profile as a function of temperature.[4] This shows that at 30°, only 4% of this molecule is in the aldehyde form.
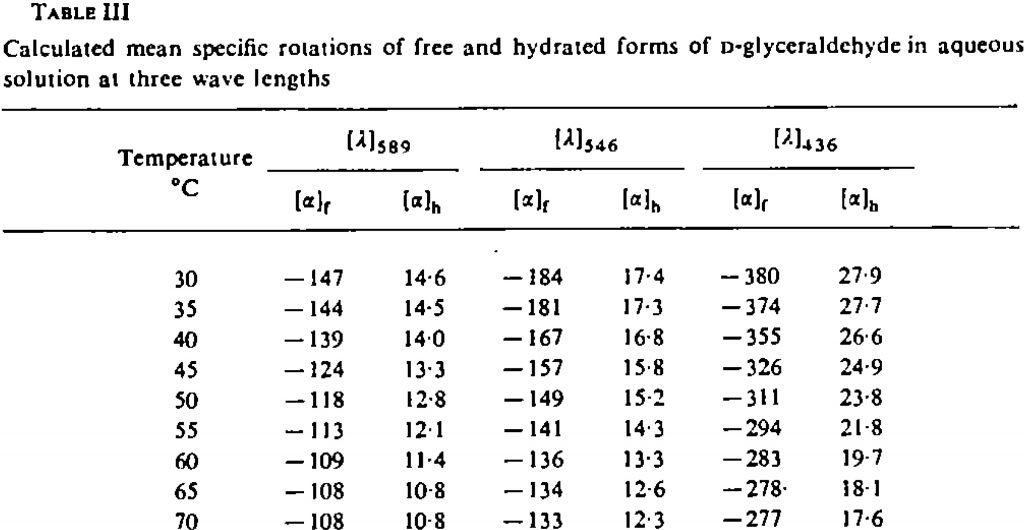
The article also reports optical rotations, at three wavelengths. I should note here that the notation (+) means only the rotation at 589nm (yes, you can get sign inversions on moving from one wavelength to another![5]). At 30° the observed rotation for D-glyceraldehyde is indeed strongly negative, whilst it is the hydrated form that is moderately (+).
There is a discrepancy of ~47° between these measured values and the calculated one at 589nm. I hope to explore the origins of this error in a separate post.
So we see by both experiment and theory that D-(+)-glyceraldehyde, if the meaning of that (+) is to be upheld, should really be D-(-)-glyceraldehyde. Note that many of the sugars shown in the top diagram have (-) as well as (+), and so there is no reason that glyceraldehyde should be any different.
References
- H.J. Lamble, M.J. Danson, D.W. Hough, and S.D. Bull, "Engineering stereocontrol into an aldolase-catalysed reaction", Chemical Communications, pp. 124, 2005. https://doi.org/10.1039/b413255f
- J.M. BIJVOET, A.F. PEERDEMAN, and A.J. van BOMMEL, "Determination of the Absolute Configuration of Optically Active Compounds by Means of X-Rays", Nature, vol. 168, pp. 271-272, 1951. https://doi.org/10.1038/168271a0
- J.G. Kirkwood, "On the Theory of Optical Rotatory Power", The Journal of Chemical Physics, vol. 5, pp. 479-491, 1937. https://doi.org/10.1063/1.1750060
- M. Fedoroňko, "Optical activity of D-glyceraldehyde in aqueous solutions", Collection of Czechoslovak Chemical Communications, vol. 49, pp. 1167-1172, 1984. https://doi.org/10.1135/cccc19841167
- M.S. Andrade, V.S. Silva, A.M. Lourenço, A.M. Lobo, and H.S. Rzepa, "Chiroptical Properties of Streptorubin B: The Synergy Between Theory and Experiment", Chirality, vol. 27, pp. 745-751, 2015. https://doi.org/10.1002/chir.22486