In the previous post, I discussed the structure of the free base form of tetrodotoxin, often represented as originally suggested by Woodward[1] below in an ionic form:
Quantum calculations suggested that this form was higher in energy than neutral forms devoid of the zwitterionic charge separation in a relatively non polar solvent such as chloroform. For this, a so-called continuum solvation model was used. But even chloroform is capable of forming rather strong O…H-C hydrogen bonds, and these specific isotropic interactions are not well modelled using a continuum solvent; you need to include the specific hydrogen bonds to do that. So here are two better models, the first including one or two chloroform molecules (in continuum chloroform) and the second three water molecules (in continuum water). The (FAIR) data for these results are available at DOI: 10.14469/hpc/6278 and you can view the 3D models by clicking on the images below.
| System | ΔΔG298, + 1CHCl3 | ΔΔG298, + 2CHCl3 | ΔΔG298, + 3H2O |
|---|---|---|---|
| Ionic form | 0.0 | 0.0 | 0.0 |
| non-ionic form | -7.1 | -4.9 | +1.5 |
Adding additional solvent molecules in a “non-stochastic” manner is clearly an approximation. For example, three water molecules added to the neutral (non-zwitterionic) form could take up residency in many different ways, given the number of oxygen atoms present in tetrodotoxin. The energies reported above are for the lowest energy forms of the two that I located; I did not investigate more possibilities. However, the ionic form, with the water molecules directly hydrogen bonding with the ionic oxygen are more certain.
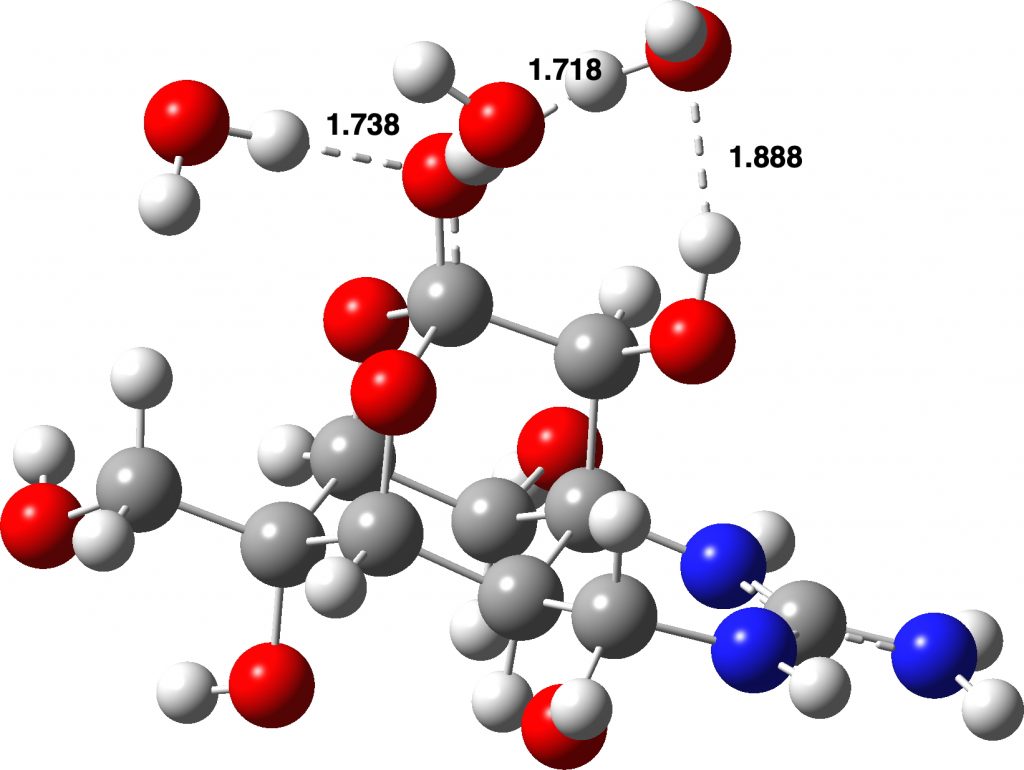
Ionic form: Click image to view 3D model
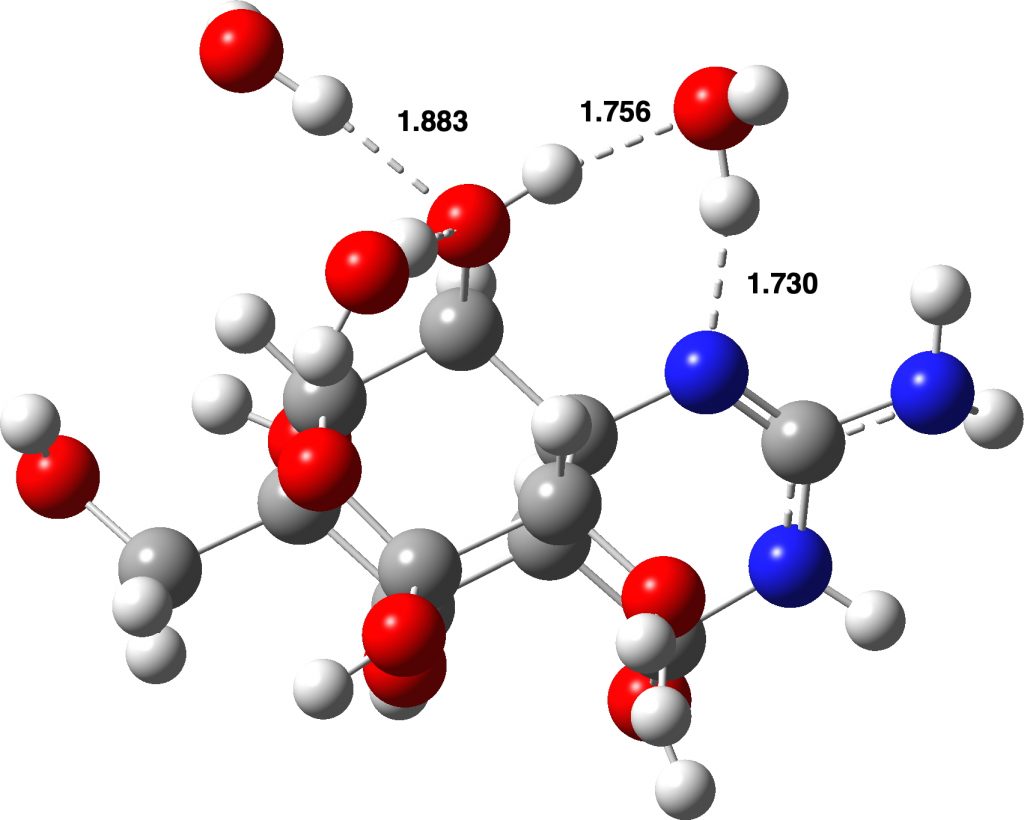
Neutral form: Click image to view 3D model
So we now see that Woodward’s original proposal for a charge-separated ionic form for the free base of tetrodotoxin may indeed be accurate, but only for polar solutions such as water. In rather less polar solutions such as chloroform it is probably not ionic. Clearly the stability also depends on the number of solvent molecules included in the model. The rather large chloroform molecule is less likely to accumulate around the ionic oxygen in numbers >>2 (and including more makes the calculation much slower), but already with just three water molecules the ionic form becomes the more stable. Also, e.g. two chloroforms or merely three waters are insufficient to form any sort of stabilizing “bridge” connecting the two ionic centres. So these results must be treated with a little caution.
It is always rather risky to bet against Woodward’s chemical intuitions and insights (as for example Robinson found when debating the structure of strychnine[2] with him). He may well have been correct with the ionic structure of tetrodotoxin as well (at least in aqueous solutions)!
References
- R.B. Woodward, "The structure of tetrodotoxin", Pure and Applied Chemistry, vol. 9, pp. 49-74, 1964. https://doi.org/10.1351/pac196409010049
- R.B. Woodward, M.P. Cava, W.D. Ollis, A. Hunger, H.U. Daeniker, and K. Schenker, "THE TOTAL SYNTHESIS OF STRYCHNINE", Journal of the American Chemical Society, vol. 76, pp. 4749-4751, 1954. https://doi.org/10.1021/ja01647a088