I previously explored stabilized “carbenes” with the formal structures (R2N)2C:, concluding that perhaps the alternative ionic representation R2N+=C–NR2 might reflect their structures better. Here I take a broader look at the “carbene” landscape before asking the question “what about nitrenes?”
The top row shows the compounds for which no crystal structure could be found.‡ This includes the traditional carbon-substituted unstabilized carbenes, as well as those substituted with either group 4A or 6A elements (Si, S, etc). Isolated hits were however found as follows for other combinations (all interesting, but I do not discuss them here).
At this point I turned to nitrenes. As with unstabilised carbenes, the nitrogen is described as having one covalent bond, one unshared spin-paired lone pair of electrons and two further unpaired electrons to give a total valence shell count of six (and in fact a triplet spin state). Are there any examples? Just one, formally corresponding to R2P-N (DEGSEP[13]). To explore what the nature of the single P-N bond is, I did a search for the P-N bond lengths of all P-N compounds in the CSD (of any bond type). The distribution shows ~1.48Å as the shortest and ~1.8Å as the longest.There is no sign of a multimodal distribution indicating partitioning into e.g. single, double or triple bonds.
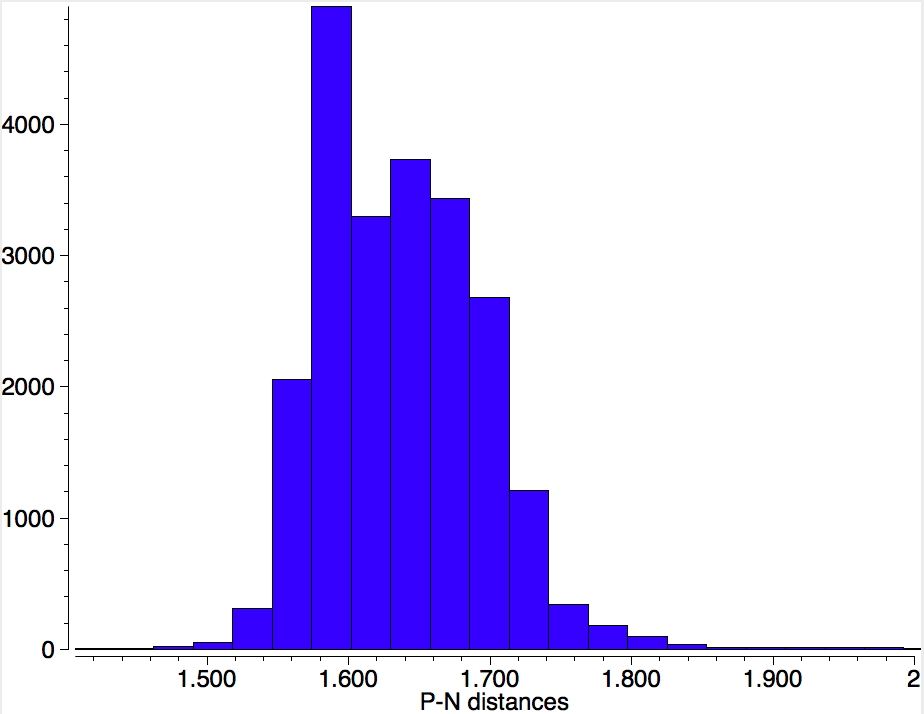
So what about our nitrene? The P-N bond is 1.456Å, which is very much at the short end of the spectrum above, and so pretty far from the formal simple definition of a nitrene given above. So now for a ωB97XD/Def2-TZVPP calculation of the singlet wavefunction[14] (the triplet state is 22 kcal/mol higher in energy[15]) for a model compound with calculated CN distance 1.493Å and from which an ELF-based localisation and integration of the electron basins can be derived. The total basin integration for the N can be taken as 7.77e (close to the octet) if basins 15 and 16 are assumed to be shared (covalent) and this gives the P-N bond double bond character. If basins 15 and 16 are not included (and taken as localised just on the P), the N has 5.81e and an associated single bond.
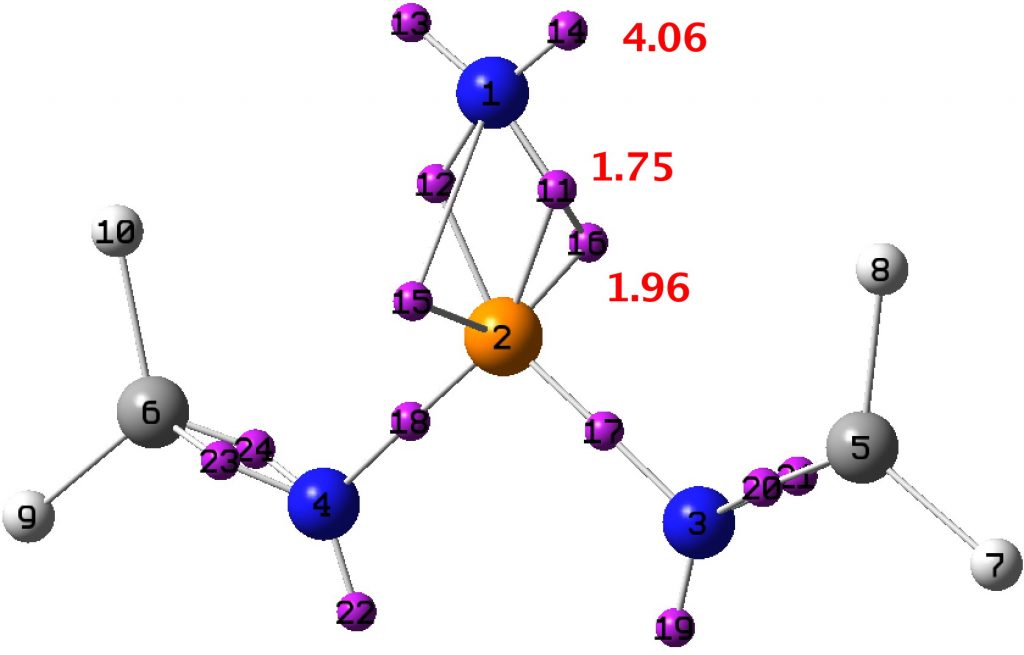
A search of the CSD specifying P≡N as the search query returns lengths in the range 1.47-1.56Å, which our example certainly conforms to. So perhaps we can tentatively conclude that the only example thus far reported of a crystalline nitrene in fact sustains a very short bond to the nitrogen. It could be considered as the N having a filled octet in its valence shell, and certainly a bond order higher than one, if not actually triple.
‡For the search query, see [16]
References
- Marchenko, Anatoliy., Koidan, Heorgyi., Hurieva, Anastasiya., Kurpiieva, Olena., Vlasenko, Yurii., Kostyuk, Aleksandr., Tubaro, Cristina., Lenarda, Anna., Biffis, Andrea., and Graiff, Claudia., "CCDC 997216: Experimental Crystal Structure Determination", 2014. https://doi.org/10.5517/cc12gp8h
- A. Marchenko, H. Koidan, A. Hurieva, O. Kurpiieva, Y. Vlasenko, A. Kostyuk, C. Tubaro, A. Lenarda, A. Biffis, and C. Graiff, "N-phosphanyl-imidazolin-2-ylidenes: Novel stable carbenes as bidentate ligands for late transition metals", Journal of Organometallic Chemistry, vol. 771, pp. 14-23, 2014. https://doi.org/10.1016/j.jorganchem.2014.05.036
- Lavallo, V.., Mafhouz, J.., Canac, Y.., Donnadieu, B.., Schoeller, W.W.., and Bertrand, G.., "CCDC 236934: Experimental Crystal Structure Determination", 2004. https://doi.org/10.5517/cc7yk1r
- V. Lavallo, J. Mafhouz, Y. Canac, B. Donnadieu, W.W. Schoeller, and G. Bertrand, "Synthesis, Reactivity, and Ligand Properties of a Stable Alkyl Carbene", Journal of the American Chemical Society, vol. 126, pp. 8670-8671, 2004. https://doi.org/10.1021/ja047503f
- Lavallo, V.., Frey, G.D.., Kousar, S.., Donnadieu, B.., and Bertrand, G.., "CCDC 651272: Experimental Crystal Structure Determination", 2008. https://doi.org/10.5517/ccpvpsz
- V. Lavallo, G.D. Frey, S. Kousar, B. Donnadieu, and G. Bertrand, "Allene formation by gold catalyzed cross-coupling of masked carbenes and vinylidenes", Proceedings of the National Academy of Sciences, vol. 104, pp. 13569-13573, 2007. https://doi.org/10.1073/pnas.0705809104
- Marsh, R.E.., and Clemente, D.A.., "CCDC 625624: Experimental Crystal Structure Determination", 2008. https://doi.org/10.5517/ccp00f3
- R.E. Marsh, and D.A. Clemente, "A survey of crystal structures published in the Journal of the American Chemical Society", Inorganica Chimica Acta, vol. 360, pp. 4017-4024, 2007. https://doi.org/10.1016/j.ica.2007.02.050
- Martin, D.., Baceiredo, A.., Gornitzka, H.., Schoeller, W.W.., and Bertrand, G.., "CCDC 252551: Experimental Crystal Structure Determination", 2005. https://doi.org/10.5517/cc8gst9
- D. Martin, A. Baceiredo, H. Gornitzka, W.W. Schoeller, and G. Bertrand, "A Stable P‐Heterocyclic Carbene", Angewandte Chemie International Edition, vol. 44, pp. 1700-1703, 2005. https://doi.org/10.1002/anie.200462239
- R.W. Alder, C.P. Butts, and A.G. Orpen, "Stable Aminooxy- and Aminothiocarbenes", Journal of the American Chemical Society, vol. 120, pp. 11526-11527, 1998. https://doi.org/10.1021/ja9819312
- A.J. Arduengo, J.R. Goerlich, and W.J. Marshall, "A Stable Thiazol‐2‐ylidene and Its Dimer", Liebigs Annalen, vol. 1997, pp. 365-374, 1997. https://doi.org/10.1002/jlac.199719970213
- Dielmann, F.., Back, O.., Henry-Ellinger, M.., Jerabek, P.., Frenking, G.., and Bertrand, G.., "CCDC 884586: Experimental Crystal Structure Determination", 2013. https://doi.org/10.5517/ccyph14
- H. Rzepa, "DEGSEP", 2016. https://doi.org/10.14469/hpc/1625
- H. Rzepa, "DEGSEP triplet", 2016. https://doi.org/10.14469/hpc/1626
- H. Rzepa, "CSD Search query for carbenes", 2016. https://doi.org/10.14469/hpc/1624
Although I drew a blank for R3C-C-CR3, there are two examples of R3C-Si-CR3; 10.5517/CC10Q5SP and 10.5517/CC4L999 which could fairly be said to be true silyenes.