The autoionization of water involves two molecules transfering a proton to give hydronium hydroxide, a process for which the free energy of reaction is well known. Here I ask what might happen with the next element along in the periodic table, F.
I have been unable to find much about the autoionization of HF in the literature; the pH of neat HF appears unreported (unlike that of H2O, which of course is 7). Even the dielectric constant of liquid HF[1],[2] seems to vary widely, the largest reported being ~84. It is suggested that liquid HF is much less ordered than e.g. water, and this suggests that a single static model is unlikely to be entirely realistic. Nonetheless, I thought it might be informative to take the model I previously constructed for water and try applying it to HF. Here is part of the geometry optimisation cycle[3] from the original edited water model. I used ωB97XD/Def2-TZVPPD/SCRF=water for the model. Why continuum water as the solvation treatment? Well, standard parameters for liquid HF are not available (perhaps given the variation in dielectric) and since the upper bound might be similar to water, I decided to use that to see what I got. Clearly however an approximation.
The low energy final geometry corresponds to 10 HF molecules and lies about 16 kcal/mol lower (in total energy) than the cyclic structure containing H2F+.F– species connected by two (HF)3 bridges and two further non-bridge HF molecules hydrogen bonding to the H2F+ and the F–. In fact the ionic structure turns out to be a transition state for proton shifting along the chain to create (HF)10, with a free energy barrier of 9.2 kcal/mol above the neutral form.[4] This difference between ionic and non-ionic forms is considerably less than that for water as previously indicated. Note also how much shorter the hydrogen bonding H…F distances are in the HF cluster.
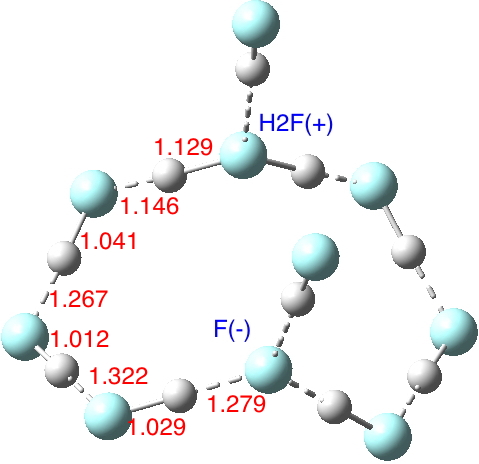
So unlike water, where the hydronium hydroxide is a clear minimum in the potential with a small but distinct barrier (~3.5 kcal/mol[5]) to proton transfer, with HF at the same level of theory the barrier is zero. Perhaps the difference might be because whereas hydronium hydroxide can support three stabilizing (H2O)3 bridges, only two (HF)3 bridges are possible with H2F+.F–. It might also be higher levels of theory (or better/larger models of the HF cluster) could well give a barrier for the process, but this does tend to suggest that the dynamics of HF liquid may suggest quite different lifetimes for autoionized forms of HF compared to water. Liquid HF is clearly just as complicated a liquid as is H2O, certainly much less is known about it.
References
- R.H. Cole, "Dielectric constant and association in liquid HF", The Journal of Chemical Physics, vol. 59, pp. 1545-1546, 1973. https://doi.org/10.1063/1.1680219
- P.H. Fries, and J. Richardi, "The solution of the Wertheim association theory for molecular liquids: Application to hydrogen fluoride", The Journal of Chemical Physics, vol. 113, pp. 9169-9179, 2000. https://doi.org/10.1063/1.1319172
- H.S. Rzepa, "H 10 F 10", 2016. https://doi.org/10.14469/ch/192032
- H.S. Rzepa, "H 10 F 10", 2016. https://doi.org/10.14469/ch/192034
- H.S. Rzepa, "H22O11", 2016. https://doi.org/10.14469/ch/192022
Tags: dielectric, energy, Equilibrium chemistry, Fluorides, free energy, free energy barrier, Hydrogen bond, Hydronium, Inorganic solvents, Lithium fluoride, low energy final geometry corresponds, Oxides, PH, Properties of water, Self-ionization of water, Water, Water model
Henry, "I have been unable to find much about the autoionization of HF in the literature; the pH of neat HF appears unreported (unlike that of H2O, which of course is 7)." Anhydrous liquid hydrogen fluoride is very strong acid, superacid. It Hammet acidity function H0 (extension of pH for strong acids) is -15. (see f.e. Olah et.al., Superacid chemistry, 2nd ed., 2009, p. 58). It is believed that the autoprotonation equilibrium of HF lies far to the right.
If the equilibrium lies to the right then the free energy of the ion-pair would be -ve compared to the un-ionized species. That would contradict my observation above that the ion-pair is a transition state (which, by definition, is higher in energy than its reactants, i.e. the un-ionized system). This could of course just mean that the true geometry for an ion-pair is different from my current model.
Overnight, one of the TS locations I had running in fact converged to a new geometry, shown below, where the ion-pair is even more apparent than previously. Although this geometry was some 5.8 kcal/mol lower in total energy than previously, its relative free energy comes out at about the same, i.e. 9.2 kcal/mol (doi: 10.14469/ch/192036) due to a commensurate large change in the entropy.
Perhaps rather than e.g. 2HF ⇌ H2F+.F– one should be looking for 3HF ⇌ H2F+.FHF–. I will commence the search!
In the context that autoprotonation equilibrium of HF lies far to the right I should note that the solid-state crystal structure is not ionized; doi: 10.1107/S0365110X54000497
I would further add that there are no instances of H-F+-H in the Cambridge structural database.
F-H-F(-) is of course quite common. Although the symmetry (or lack of it) of the bond can vary a lot, the centroid does appear to correspond to a symmetric bond.
The following structure (doi: 10.5517/CC1JYSGQ) shows the FH…F–…HF motif found in the structure shown in the post.
In the crystal structure of solid HF noted above, the F…H…F hydrogen bonds are recorded as having the length 2.49 (F…F distance, and a bit longer than ~the sum of the two F-H distances shown above at the red "hot-spot") with an unsymmetrical positioning of the hydrogen. The F(-)/H2F(+) alternative was analysed but found to be less probable. A modern analysis would certainly help clear things up.
I have done a slightly more elaborate search of the CSD for F(-), surrounded by non-bonded contacts to a H (i.e. H-bond), and excluding any hits arising from e.g. F-Metal or F-non-metal (this because one cannot specify the number of bonds to the F as zero, the minimum being 1). The search query is at doi: 10.14469/hpc/440) As of April, 2016, 165 hits were found.
The heat plot below shows two (of the three defined F…H distances; in fact all three combinations of two distances show similar distributions. The "hot-spot" occurs at F…H distances of around 1.9Å.
I have picked three individual structures, all of which look interesting by virtue of the shortness of the F…H distances. The first of these shows 6-coordinate fluoride anion, which is surprisingly large given how small the anion is.
While liquid HF is indeed a strong acid, this fact does not translate into a large autodissociation. Values for pK(HF) in the literature are 10E-10.7 (273 K, found in Holleman-Wiberg) or 2E10-12 (298 K, found in www), they are thus below the value of water.
I meant K(HF), not pK(HF).
…and thus higher than Kw of water… sorry.
Lukas, could you clarify? 10E-10.7 is 1E-9.7 and 2E10-12 is 1E-9.7 or 1E-11.7? So pKw water is 14 and HF is 9.7 or 11.7, both of which values are less than water (more autoionized). I thus make the free energy difference between the ionic and non-ionic forms ~13.22 or 15.95 kcal/mol, indeed lower in energy than that of water.