These posts contain the computed potential energy surfaces for a fair few “text-book” reactions. Here I chart the course of the cyclopropanation of alkenes using the Simmons-Smith reagent,[1] as prepared from di-iodomethane using zinc metal insertion into a C-I bond.
Two reactions it can be compared with are the epoxidation of ethene using a peracid and dichlorocyclopropanation. The latter is a four-electron pericyclic process, which is thermally forbidden. The outcome there is that the two new bonds form very asynchronously to avoid transition state antiaromaticity. The former is a more complex reaction, best described as a six-electron process which allows both C…O bonds to form at the same rate. So which of these two does the Simmons–Smith mechanism correspond to?
The calculation as undertaken at a ωB97XD/Def2-TZVPD-PP (solvent=dichloromethane) level[2] shows the two C…C bonds forming at more or less the same rate. The reaction therefore resembles epoxidation rather than dichlorocyclopropanation.
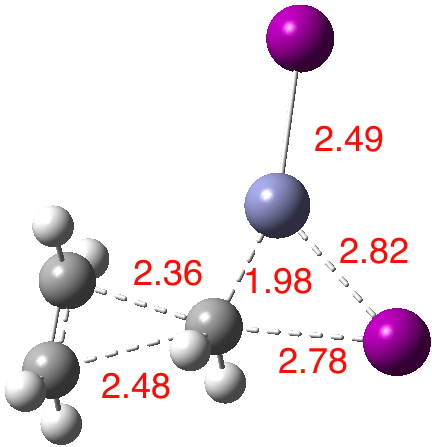
The intrinsic reaction coordinate (IRC)[3] is shown below, revealing a concerted and almost synchronous reaction. The synchronicity is all the more surprising given the diversity of bonds forming/breaking.
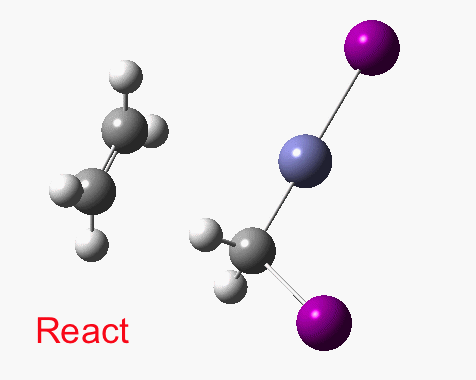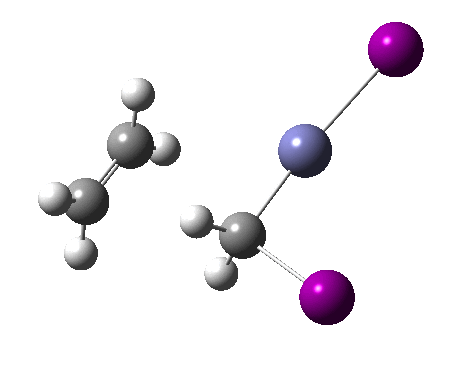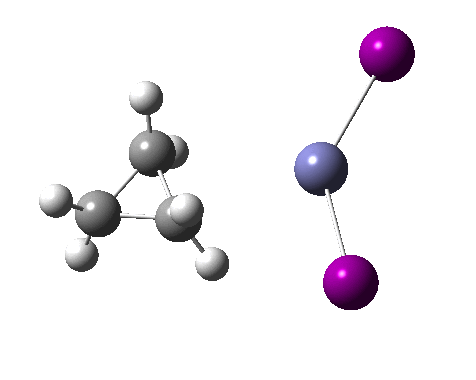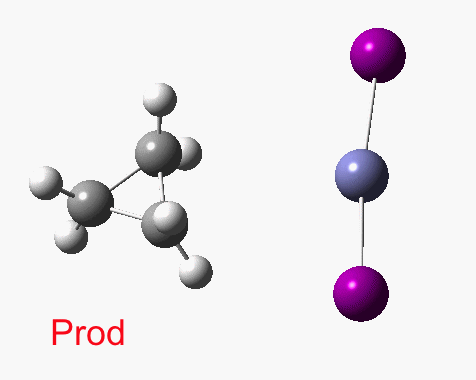
There are slight and tantalizing hints that the alkene and the I-Zn-CH2-I components initially form a weak π-complex, which then rearranges into the TS, and then again a weak complex at the end. The NCI surfaces for both are shown below, and show clear signs of dispersion-like stabilisations for both of them! The strange torus around the Zn-I bond is due to the need for a lower density threshold to filter out the covalent interactions.
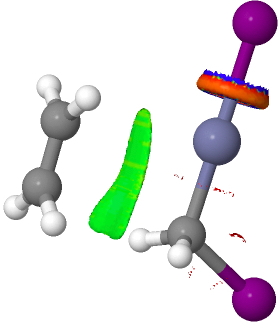
Click for 3D

Click for 3D
References
- H.E. Simmons, and R.D. Smith, "A NEW SYNTHESIS OF CYCLOPROPANES FROM OLEFINS", Journal of the American Chemical Society, vol. 80, pp. 5323-5324, 1958. https://doi.org/10.1021/ja01552a080
- H.S. Rzepa, "C 3 H 6 I 2 Zn 1", 2014. https://doi.org/10.14469/ch/147617
- H.S. Rzepa, "Gaussian Job Archive for C3H6I2Zn", 2014. https://doi.org/10.6084/m9.figshare.1270441
Tags: computed potential energy surfaces, di-iodomethane using zinc metal insertion, Simmons
Here is a binuclear variation of the same reaction.