The following is a short question in a problem sheet associated with introductory organic chemistry.
- Q: “Show curly arrows for the formation of the product of the following reaction, together with a Lewis representation of that product: Et2O + MgBr2“.
- A: Et2O+-Mg–Br2 (a product by the way that is known as magnesium bromide ethyl etherate, and which is commercially available as a solution).
First a few tutor-like comments. The Mg is tri-coordinate in this simple representation, and if we assume that the bonds are covalent, has six electrons in the Mg valence shell. In modern notation, the Mg has a formal charge of -1 and the oxygen +1. The Mg thus does not have a filled 3s/3p valence shell, which would be eight. But few (students or tutors) go on to apply a reality check. So here is one.
The reality check involves a search for a crystal structure, which is really trivial to set up. And what we find are the following.
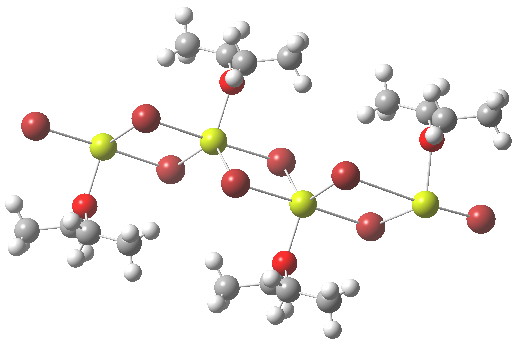
- The first hit with exactly this stoichiometry has the CCDC code TOQKIT and a polymeric structure as below. Each Mg is coordinated by four (bridged) bromines and one oxygen, giving trigonal bipyramidal penta-coordination. The valence electron count at Mg is now eight, but distributed around five bonds, not four. Since we no longer have formal Lewis two-electron covalent bonds, it is difficult to assign a Lewis-like charge to the atoms.
Click for 3D
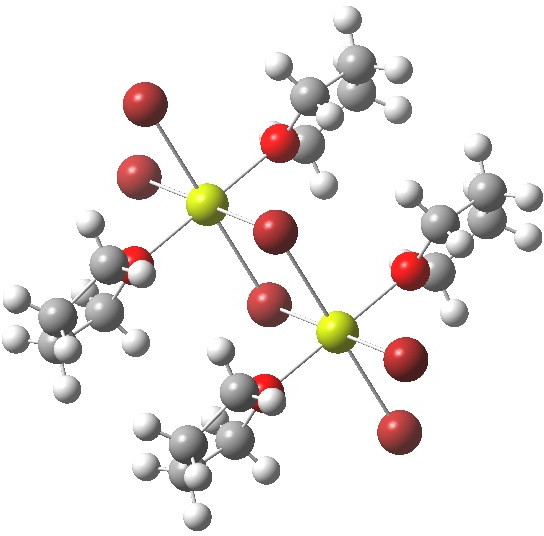
- The next hit actually corresponds to the stoichiometry 2R2O + MgBr2 (R=thf). This again is polymeric, but differs from the first structure in having octahedral Mg (six coordination).
Click for 3D.
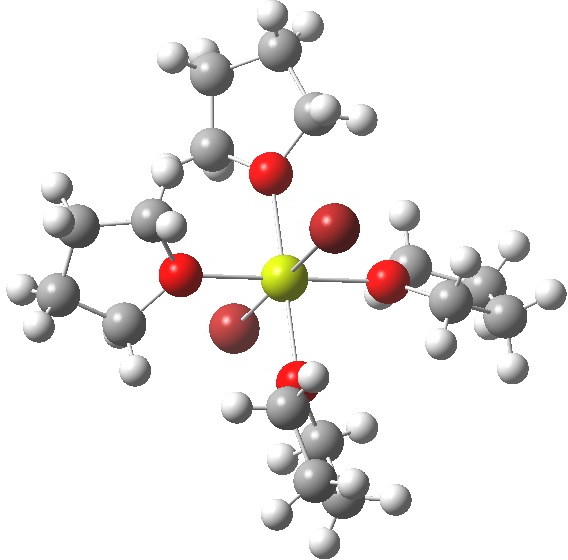
- OK, even more ether: 4R2O + MgBr2. Finally, non polymeric but again with six-coordinate octahedral Mg. The Mg again has a filled valence octet, and again the bonds are not two-electron ones, hence no charges are attempted. So just a change in the stoichiometry can result in fascinating changes to the resulting structure.
Click for 3D
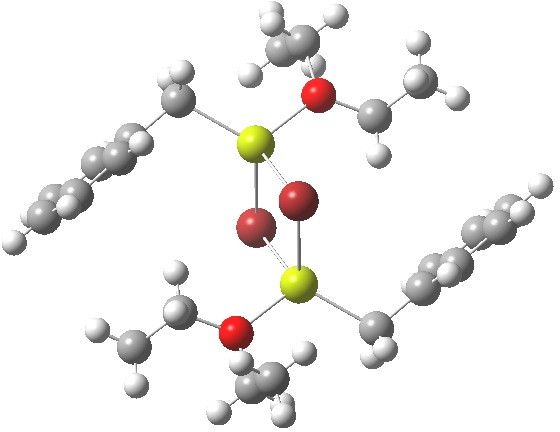
- Finally, a variation; benzyl magnesium bromide (a Grignard reagent) shows tetrahedral coordination.
Click for 3D
Students (and tutors) who get as far as this are amply rewarded I hope!
Tags: Grignard, octahedral, pence, pentagonalbipyramid, tetrahedral
Could you please go into more detail about how you arrived at the electron count of 8 around each Mg in Et2O-MgBr2, and how you know the bonds are not the standard-issue 2 electron bonds? Is this a calculation or was it measured in the Xray structure? Thanks.
For monomeric Et2O-MgBr2 the formal electron count around each Mg is six, not eight. I presume you meant the polymeric structure, where the Mg is five-coordinate. If each of these bonds were to be standard issue 2-electron Lewis models, that would then give the Mg a valence count of 10. This is energetically far to high and is never observed. Eight is the absolute maximum for all main group elements, including the early groups (such as Mg) and indeed the late groups such as Cl or I (see this post or this one). I note that there does tend to be a fixation on electron-pairs, as in for example valence-shell-electron-pair-repulsion theory (VSEPR). But a shared-electron bond can form with fewer electrons!
But Mg does normally manage to achieve 8, as in Ph2Mg.
Most of our information about electron density comes from X-ray measurements of solid compounds. These routine measurements measure the electron density around a nucleus. There is a specialised technique, involving high-angle scattering which tries to also measure the electron density in a bond, but this is difficult and is rarely done. Perhaps if such bond measurements were to be more frequently made, we would learn a lot more about bonds. Meanwhile we rely on calculations. Where a direct comparison of bond electron density between calculation and measurement has been made, the agreement is pretty good. So I think we can probably trust calculations most of the time.