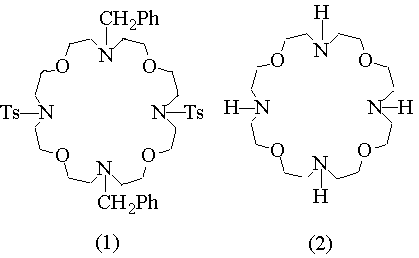
In previous studies concerning the synthesis of new macropolicyclic lipophilic receptors selective for Na cation (ref. 1), we have isolated, in 5% yield only, a very interesting compound, namely 4,16-bis(p-toluensolphonyl)- 10,22-bisbenzyl- 1,7,13,19-tetraoxa- 4,10,16,22- tetraazacyclotetracosane (1). Besides the presence of four oxygen and four nitrogen alternate binding sites, this macrocycle feature the opposite nitrogen atoms protected by tosyl and benzyl groups. The possibility of selective nitrogen deprotection makes compound (1) a suitable building block for the synthesis of more sophisticated polycyclic receptors with new topological arrangements of binding sites, like 26 - (BENZYL)PROPENYL - 4, 10, 16, 22 - TETRAOXA - 1, 7, 13, 19 - TETRAAZA - (12.12.3) - TRICYCLOHEPTACOSANE (4). In order to have larger quantities of this material we devised a new synthetic procedure which affords (1) in 45% yield after column chromatography.
Debenzylation of (1) with hydrogen and Pd/C-5% as catalyst in acetic acid as solvent, and condensation of the resulting bis-amine with 2-benzyl- 1,3-propandiol bis-p-toluensulphonate afforded the bicyclic bis-tosylamido derivative which, after reductive detosylation by lithium aluminum hydride in THF gave the title compound in 30% overall yield. This compound proved to be very effective in the solubilization of ammonium salts in non-polar solvents, while the complexation capability is quite low in the case of alkali metal salts.The extent of complexation of (4) with several ammonium salts, including aminoacid esters hydrochlorides and alkali salts and, for comparison, the data obtained using bis-N-benzyl (3) and fully deprotected macrocycle (2) as ligands, are reported.