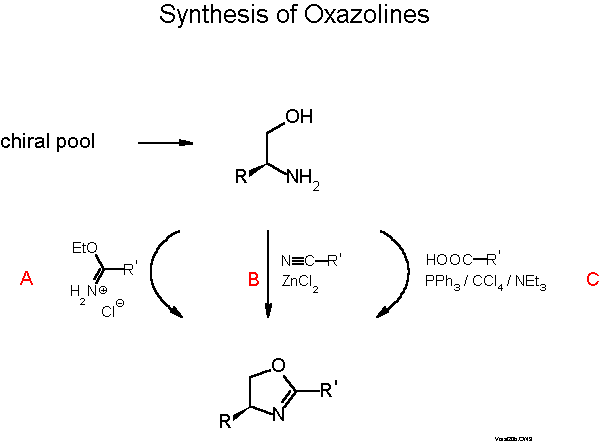
The oxazoline moiety is available from amino alcohols, which in turn can be prepared from the chiral pool of natural amino acids. There are many convenient routes from amino alcohols to oxazolines that Gant and Meyers have very recently reviewed in a Tetrahedron Report[13]. Most people prefer one step procedures as described in Scheme 6.

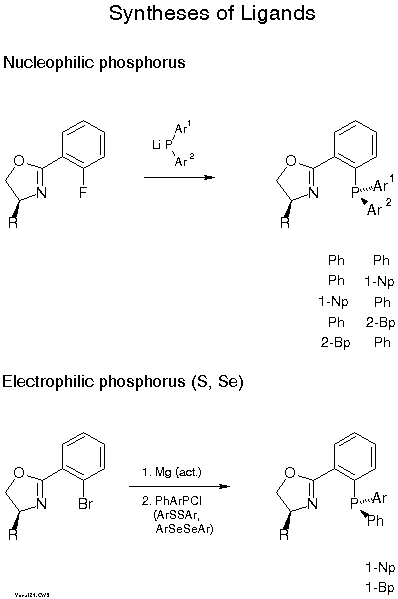
We normally use the one-pot condensation (C) with a carboxylic acid under conditions (triphenylphosphine/CCl4/base) developed by Vorbrueggen[14]. Yields are typically 40-50 %. Higher yields are achieved with a three step procedure involving (i) formation of an N-acyl amino alcohol, ( ii) activation of the OH group and (iii) ring closure with base[15]. The Pfaltz[16] and Williams[17] groups prefer the procedure B developed by Witte and Seeliger[18]. Scheme 7 describes introduction of phosphorus. The most convenient way is nucleophilic substitution of fluorine with a diarylphosphide which proceeds with 70-90 % yield[19][11b]. In the case of stereogenic phosphorus, with, e.g., Ph and 1-naphthyl or 2-biphenylyl, 7:3 mixtures of diastereomers are formed which can be easily separated by flash chromatography or crystallization. Electrophilic phosphorus and also sulfur and selenium compounds can be reacted with the Grignard compound obtained from the bromo derivative and activated Mg. Yields with halophosphines are only 30-50 %, but only one of the P-diastereomers is formed with selectivity of >85:15 for the examples given in Scheme 7.