The rather close parallelism between isomer ratios of the pi-allyl complexes and enantioselectivities is very satisfactory as it is in excellent agreement with our mechanistic assumptions. On the other hand, the production of precisely racemic compounds in the case of the cyclic substrate is somewhat unsatisfactory from a preparative point of view. Clearly, a ligand was required that reaches into the narrow area directly under or over the allylic atoms C-1,C-2,C-3. Such a ligand is perhaps the biphenylyl derivative displayed in Scheme 20[24].
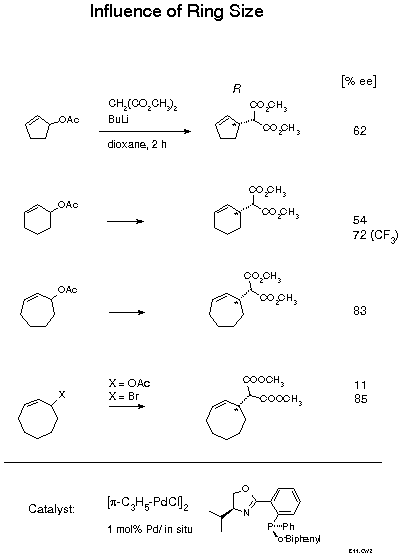
The biphenyl substituted ligand is quite effective with cyclic substrates. Best results were achieved with lithium malonate as nucleophile and dioxane as solvent. The effectiveness of lithium is remarkable as with modular diphosphines particularly good results were obtained with tetraalkylammonium salts[25]. Enantioselectivity is a function of ring size. With the very slim cyclopentenyl and cyclohexenyl substrates, enantiomeric excess near 60 % was obtained. This result was recently improved to 72 %ee by introducing two electron withdrawing m-CF3 substituents into the phenyl group. The larger and, therefore, broader 7- and 8-membered rings gave higher enantioselectivities of 83 and 85 %ee, respectively. In the latter case, a remarkable effect of the leaving group was observed, with the acetate only 11 %ee, with the bromide 85 %ee. These cycloalkenylmalonates are of interest as starting materials for natural products syntheses. As the selectivity with oxazolinophosphines could not be improved beyond the level described, we have recently developed new ligands that allow up to 99 %ee to be achieved[26].
