How about other nucleophiles? We and others have investigated, in addition to malonates, a variety of different nucleophiles: amines and n-acylamides[27], nitro compounds[28], and p-toluenesulfinate[29]. As a rule, these nucleophiles are less reactive than malonates; however, enantioselectivities are very similar which is in accord with mechanistic proposals previously discussed.
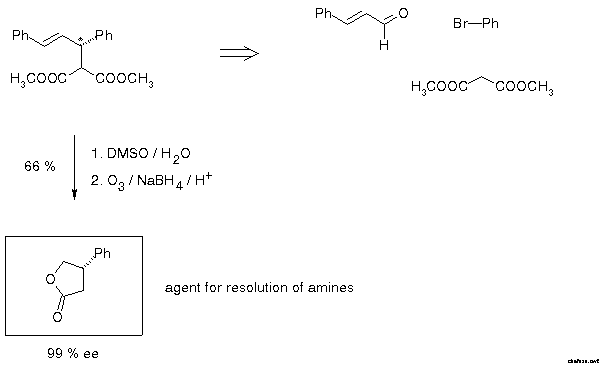
In 1979 this group developed a method for enantiomer resolution of chiral amines which was based on the formation of diastereomeric amides by heating the racemic amine with enantiomerically pure 3-phenylbutyrolactone[30]. At that time there was no convenient way to prepare this compound. It was gratifying that the standard example of allylic alkylation opened the straightforward access described in Scheme 21.

Cyclic, in particular 5-membered allylic derivatives as described in the previous section are starting materials. For many applications, however, there were initially two problems preventing applications: first, enantiomerically pure compounds were needed, and secondly, applications required a prohibitively large amount of precious material, for a 1 mol batch of ca. 150 g of product, an amount of 1 mol% of catalyst means ca. 5 g of the chiral ligand. Our solution of these problems[31] is shown in Scheme 22. The first problem could be very easily solved by using a more reactive starting material, the chloride instead of the acetate. Cyclopentenyl chloride is available by simply treating cyclopentadiene with HCl gas. High reactivity of this compound allowed the amount of catalyst to be reduced from the customary 1-3 mol% to a really satisfactory 0.02 mol% which corresponds to a turnover number of 5000, per ca. 3 hours. Enantiomerically pure material could be obtained by saponification, decarboxylation and reaction with iodine to give the iodolactone which is obtained enantiomerically pure with remarkable ease by recrystallization.
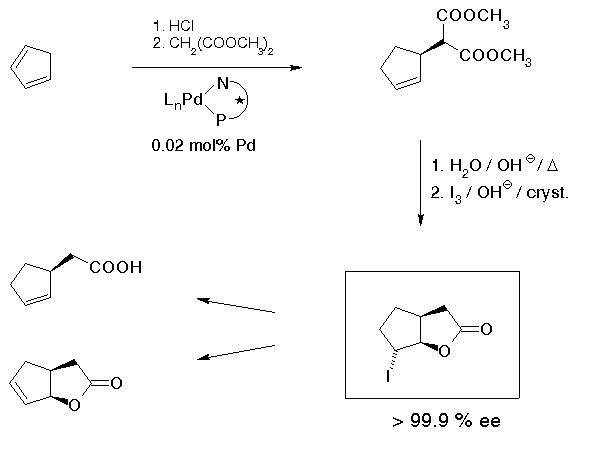
This iodolactone can be transformed into a variety of useful compounds; for example, cyclopentenyl acetic acid that has been used for a synthesis of chaulmoogric acid[32] which has been used in treatment of leprosy.