This is the follow-up to the previous post exploring a typical nucleophilic addition-elimination reaction. Here is the elimination step, which as before requires proton transfers. We again adopt a cyclic mechanism to try to avoid the build up of charge separation during those proton movements.
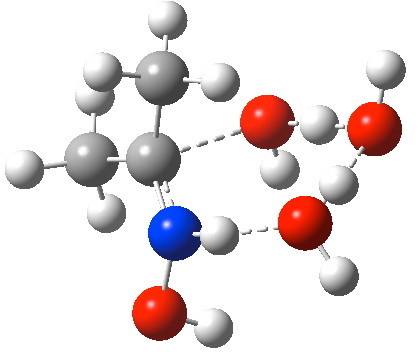
Elimination step to form an oxime. Click for animation of reaction mode.
- Overall, the transition state for this second stage is 12.1 kcal/mol higher in free energy than the addition step described previously, and some 30 kcal/mol starting from the tetrahedral intermediate. Unlike the first step, where neutral water could participate in a reaction with a low barrier, here neutral water does not do the trick. To reduce the barrier, one probably needs to add e.g. an acid, say HCl to the components. Knowing precisely where to place such an acid is non trivial – I will not reveal an answer now, but will reserve it for a future post.
- It is worth observing the features (best seen in the gradient norm plot below) in the IRC. The small feature at IRC -9 corresponds to rotation of the C-OH group away from an anomeric conformation to prepare it for accepting a proton.
- By IRC -2 (i.e. before the actual transition state is reached), the first proton transfer is well under way from the C-OH group to the first water molecule. The cleavage of the C-OH bond is also starting.
- At the transition state (IRC =0.0), this first transfer is almost complete and the second between the two water molecules is starting. The cleavage of the C-OH bond is largely complete.
- By IRC +3, this second transfer is largely complete and a third from the N-H to the second water is underway.
- By IRC +5, the proton transfers are all finished, and the C=N double bond of the oxime is also largely formed.
Well, this particular sequence of events is clearly not the full (or even a partial) answer to the mechanism of the second elimination step for this reaction. We know this because it predicts far too large a barrier. Something is missing from this model, and that something is probably a polarizing group such as HCl. Watch this space.
[…] Oxime formation from hydroxylamine and ketone. Part 2: Elimination. […]
Good afternoon
I’m performing this reaction and everything occurs very well, but I have one question that no paper I have read ansewered: why does the cis isomer is formed rather than trans? In my reaction I have a ratio of 85:15 of cis over trans. I have read a lot of papers but all f them show that this result is the usual but none of them gives an explanation. If you could help me it wuld be great.
Thank you
You would have to specify what the two substituents are that enable cis and trans to be differentiated. Only then could e.g. an 85:15 ratio be explained.
The reaction is with 2-(non-8-enyl)-cyclopentanone, and then I get the cis prefered product of the oxime. The papers I have been reading shows that usually with the cyclics systems the cis product is formed preferentially over trans but there are no explanation why. Thank you for your help.