Semibullvalene is a molecule which undergoes a facile [3,3] sigmatropic shift. So facile that it appears this equilibrium can be frozen out at the transition state if suitable substituents are used. This is a six-electron process, which leads to one of those homologous questions; what happens with ten electrons?
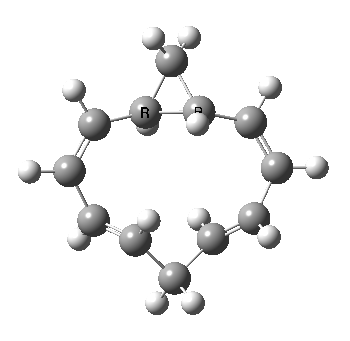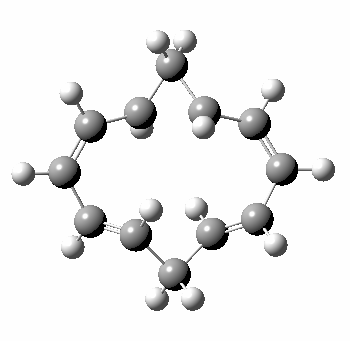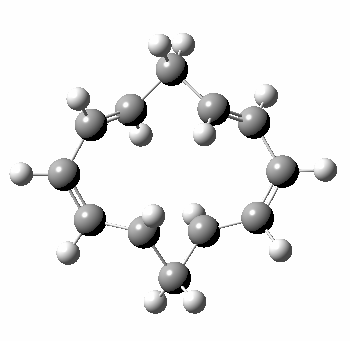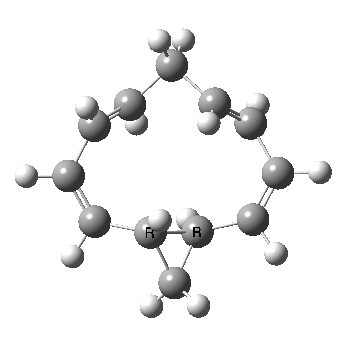
A 5,5 double Möbius sigmatropic rearrangement. Click for 3D model.
The carbocyclic version (X=CH) is a true [5,5] sigmatropic, ten electron reaction. This one has a twist however. Two in fact; I have shown it as a double-Möbius version, with two antarafacial components! Inspect the model to verify this for yourself. Such systems also have the potential to be aromatic. As you can see from the IRC pathway shown below, the activation barrier is quite high (but not unfeasible so for a thermally activated reaction). To freeze it out (i.e. to remove the barrier entirely) we have some work to do.
Well, I used the same trick as previously; turning to the tetra-aza derivative (X=N) and for good measure adding two additional cyano groups. As you can see below, this did reduce the barrier, but it’s still a long way from becoming zero. Still, [5,5] sigmatropic shifts are not exactly thick on the ground, and double-Möbius versions rarer still! The substituted one should have a low enough barrier to observe fluxional NMR behaviour at around room temperatures. If no other process takes over of course!
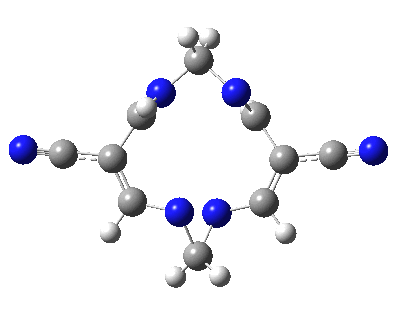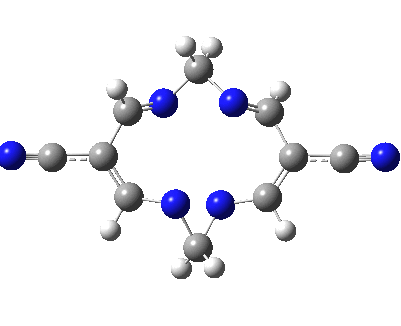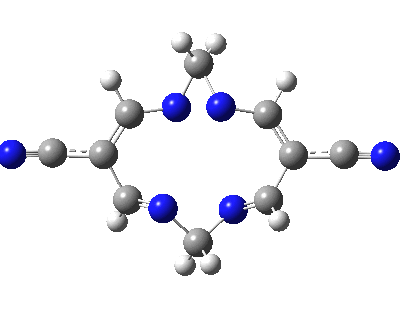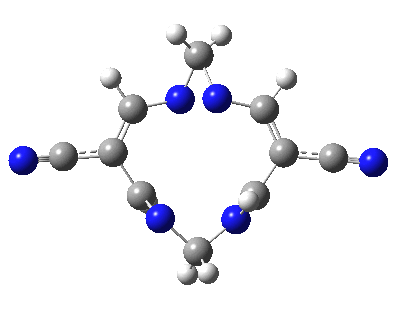
A substituted [5,5] sigmatropic rearrangement. Click for 3D model.
It is also worth noting that ten-electron aromatic systems are not so stable as six-electron ones (as Clar observed), and so suppressing any six-electron pericyclic reactions becomes the challenge.
Tags: aromatic systems, Clar, pericyclic, Reaction Mechanism
[…] is claimed to be an example of the quite rare [5,5] sigmatropic migration[1], which is a ten-electron homologation of the very common [3,3] sigmatropic reaction (e.g. the Cope or Claisen). Some benzidine […]