The four-electron thermal cycloaddition (in reverse a cheletropic elimination) of dichlorocarbene to ethene is a classic example of a forbidden pericyclic process taking a roundabout route to avoid directly violating the Woodward-Hoffmann rules. However, a thermal six-electron process normally does take the direct route, as in for example the Diels-Alder cycloaddition as Houk and co have recently showed using molecular dynamics[1]. So can one contrive a six-electron cycloaddition involving dichlorocarbene?
Surely, it should now form the two new C-C bonds at the same time (synchronously)? Well, here comes a ωB97XD/6-311G(d,p)/SCRF=dichloromethane intrinsic reaction coordinate calculation:
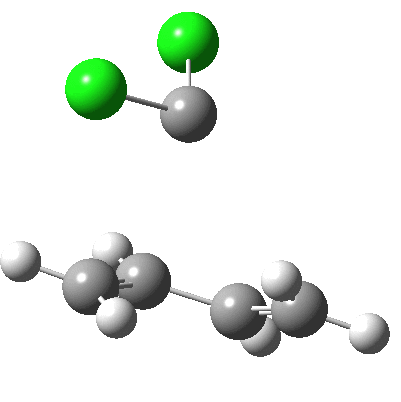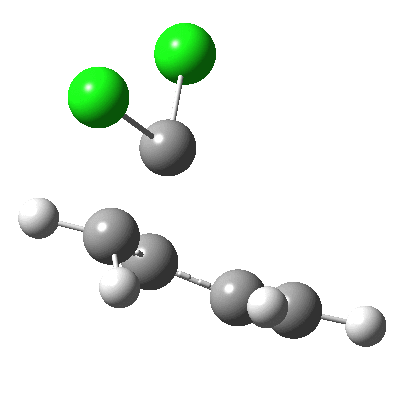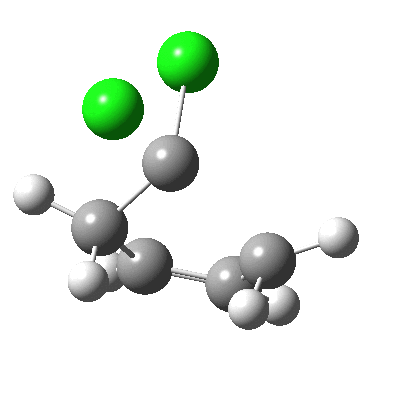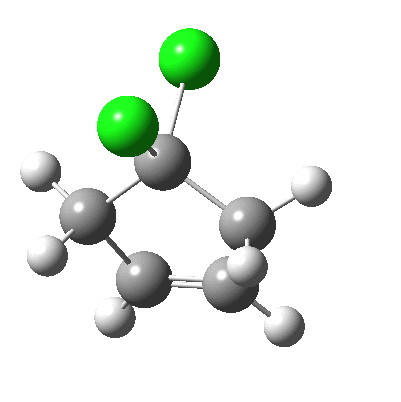
Butadiene + dichlorocarbene.
- The reaction starts at IRC -5,
- and proceeds with only a small barrier to the transition state (IRC =0.0)
- At IRC +4, the potential flattens out and the gradients drop, with formation of the first C-C bond completed. But the gradients do not quite go to zero, which would have implied the formation of a discrete intermediate such as:
- The concerted reaction continues and by IRC ~ +11, the two chlorine atoms now exhibit quite different C-Cl lengths. The one that is orthogonal to the second forming C-C bond is normal (1.815Å), whereas the one antiperiplanar to the C-C bond is 1.92Å. There are some interesting stereoelectronic alignments involved.
- Coincidentally perhaps, but these phenomena of an intermediate almost forming in a system containing a CCl2 group with concomitant lengthening of one C-Cl bond compared to the other, was also observed in my IRC for the addition of thiolate to a dichlorobuteneone. For that system, Dan Singleton’s work had shown that molecular dynamics is necessary to obtain a more complete picture, and that may well be also true for the example here! Perhaps Ken Houk might give it a go!
- The second C-C bond then completes at around IRC +16.
Well, this shows that a reaction only modestly removed from the classical six-electron Diels-Alder can change character dramatically from the synchrony expected of the latter. I am hunting for a simple explanation of this phenomenon, but perhaps participation of the C-Cl bonds makes this different from a simple cycloaddition. Or possibly, the explanation will only properly emerge when the molecular dynamics is studied?
References
- K. Black, P. Liu, L. Xu, C. Doubleday, and K.N. Houk, "Dynamics, transition states, and timing of bond formation in Diels–Alder reactions", Proceedings of the National Academy of Sciences, vol. 109, pp. 12860-12865, 2012. https://doi.org/10.1073/pnas.1209316109
Tags: asynchronous, Houk and co, pericyclic, Reaction Mechanism
[…] addition of dichlorocarbene to butadiene (see this blog for […]