The mechanism of the reaction of alkenes known as ozonolysis was first set out in its modern form by Criegee. The crucial steps, (a), (b) and (d), are all pericyclic cycloaddition/eliminations. The last step (e) is known as reductive ozonolysis, and this step is often treated as an afterthought, part of the work-up of the reaction if you like (it is not illustrated in Criegee’s review for example). Here, I will attempt to show that it is actually a very interesting mechanistic step.
Step (e) reminded me of a mechanism we had recently investigated, involving desulfurization of epidithiodioxopiperazine fungal metabolites in which an S-S bridge is reduced by one sulfur atom by the action of triphenyl phosphine. Dimethyl sulfide in turn is used to reduce a O-O fragment of the trioxolane intermediate produced from ozonolysis by one oxygen. The transition state for path (e) is shown below (ωB97XD/6-311G(d)/SCRF=dichloromethane).
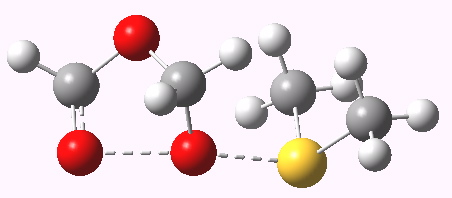
Transition state geometry for step (e). Click for 3D.
The reaction IRC for this step is shown below
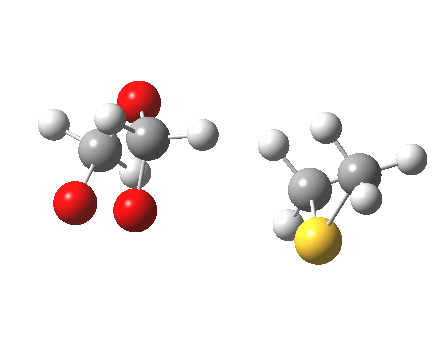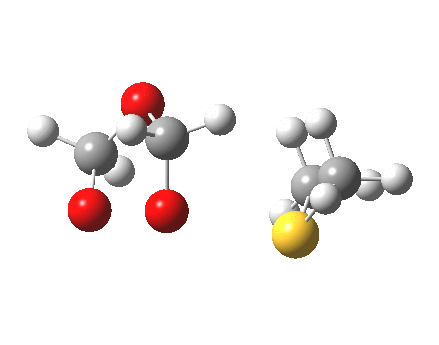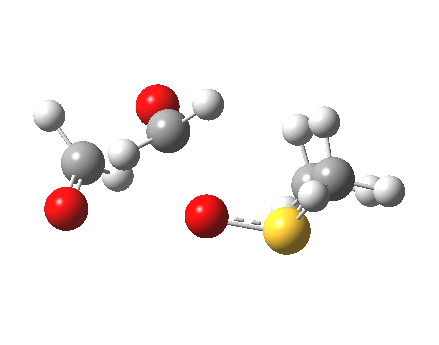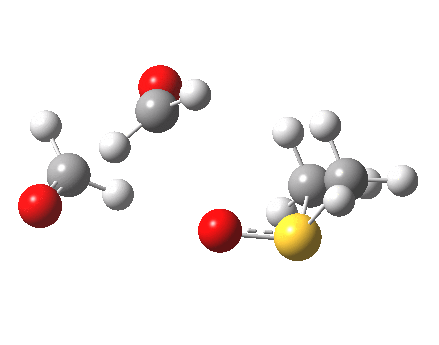 |
|
- Notice how the sulfur atom approaches more or less along the axis of the O-O bond.
- At the transition state (IRC=0) the three atoms are almost collinear. This might have some steric consequences for sterically congested alkenes.
- At IRC = -4, cleavage of the O-O bond is almost complete, an the system is starting to resemble the zwitterion shown below (the calculation is done with a solvent continuum field applied, to help stabilise any ionic intermediates that might form). One might be tempted to ask how this species could be stabilised to the extent of having a less transient existence.
- Still, it is highly transient, since there is no actual minimum in the IRC energy profile. Instead, between IRC -4 and -15, the remaining bonds cleave to form the final product shown in the scheme above. As with the previous post, which illustrated the Baeyer-Villiger rearrangement, this reductive elimination is also very asynchronous, with the pair of C-O bonds cleaving after the O-O.
This again illustrates the reactions where several bonds are either forming or cleaving, the relative dynamics can be quite unpredictable. It may even be strongly influenced by substituents and solvation. All text books of organic chemistry I know of rarely if ever address this aspect of mechanism. With new generations of interactive and dynamic text books about to spring upon us, it might be time to rethink what goes into them. I would hope it is not just a rehash of what one might call the classical arrow pushing representations of mechanism.
Tags: 200th post, Historical, pericyclic, S bridge
Any chance we could get the reaction schemes and graphs in a png or jpg format, with maybe a link to the svg? Unless I click on them they don’t show up in firefox.
Thanks!
Most recent versions of Firefox support SVG directly, so can you try updating your Firefox? Firefox 12, Chrome 18, Opera 11, Safari 5 all support SVG natively. Internet Explorer 9 has preliminary support, and no doubt IE 10 will have full support. Devices such as the iPad/iPhone also fully support SVG.
Ahh, that did it. Thanks.
I include here stitched IRCs for steps a + b followed by b + c of the ozonolysis for completeness.