A feature of many a classic review article is that not only does it organise and rationalise existing literature, but it will predict new chemistry as well. I have already noted Woodward and Hoffmann’s (WH) review as achieving the former, and here I take a (sideways) look at one of their predictions.
Compound 286 was imagined by them as a π2+ π2+ π2+ π2 cycloaddition sustaining four antarafacial bond formations across the four bonds labelled a. To really see this is true, you have to look at a 3D model (if you can see this without a model, you must have a superb stereochemical brain) and inspect how the bonds form with respect to any of the four alkene units. What other patterns are perceptible?
- Four electron pairs are involved in the reaction. In terms of aromaticity, this is a 4n electron process, and for an even (or zero) number of antarafacial components, this would be anti-aromatic transition state.
- Following their rules, WH suggested that the instead, the photochemical cyclisation would be an allowed pericyclic process. Clearly, attempts were made to synthesise 286 to test this hypothesis, but they appear to have been unsuccessful and so the prediction remains unproven.
- The arrow pushing shown for 286 also reminds that it could be considered as a homo(anti)aromatic molecule, with the circulation of arrows being reminiscent of how the two resonance forms of benzene can be interconverted. In this case, the arrows circulate to the dashed lines, where no pre-existing σ-bond is already there (a homo-conjugated motif).
- Putting all this together, I decided that the molecule on the right might be interesting in its own right. This is currently unknown (either by synthesis, or by calculation). By virtue of having an additional double bond, one converts it to a 4n+2 electron system. This now is an allowed thermal pericyclic process with the following aspects:
- All three alkenes and the butadiene motif would still form their bonds antarafacially.
- This could also be regarded as a quadruple homoaromatic system
- One can ask how synchronous the formation of the four new σ-bonds might be?
- And in this regard, six electrons have no problem moving synchronously, but what about ten?
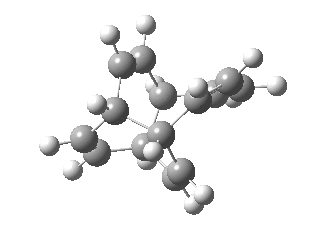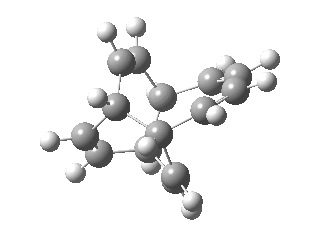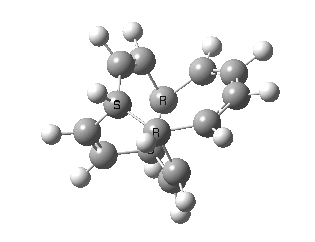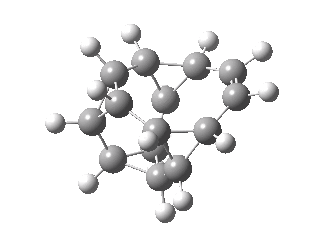
Well, here is the calculated transition state for the reaction. It actually has C2 symmetry and so is a chiral geometry.
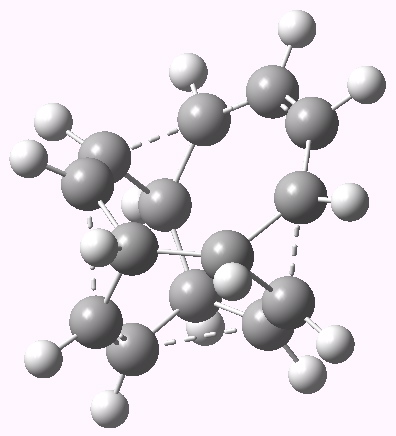
Transition state for 10 electron circulation. Click for 3D.
Sadly, as the IRC below shows, the free energy activation barrier for the (endothermic) reaction is not thermally accessible, and so there would be little point to encourage anyone to try to synthesise this species. But it certainly shows that even hypothetical reactions like this can have interesting features worth learning about.
| – |
Tags: pericyclic
Does the barrier drop significantly if one of the alkene moieties is replaced by an allyl cation moiety [thus retaining the 10 e- circuit]?
I hope it isn’t a problem to submit a comment 7 years after the original post. I just found it.
Re: 7 years; its no problem at all. I have indeed noticed that unlike many a blog topic relating to “social media”, chemistry tends to have a rather longer relevant “shelf life”.
Regarding replacing ethene with a 3-carbon two electron unit, its an interesting suggestion. The coordinates for the TS are hyperlinked above to a data repository, so the “pain of entry” should be lower than it would normally be. If I have time I might give it a go, but also encourage others to try this sort of “experiment” out.