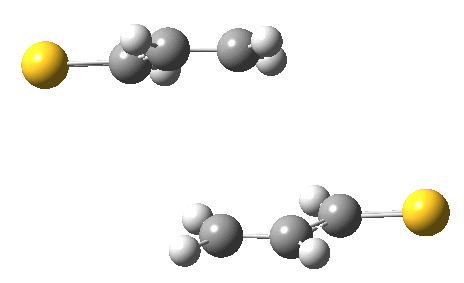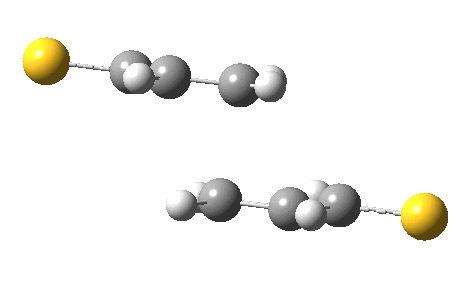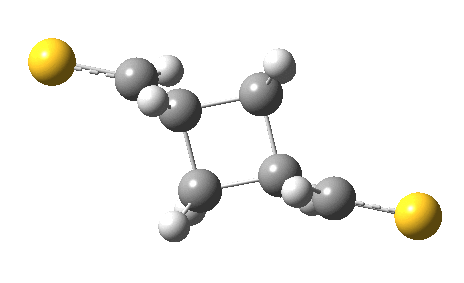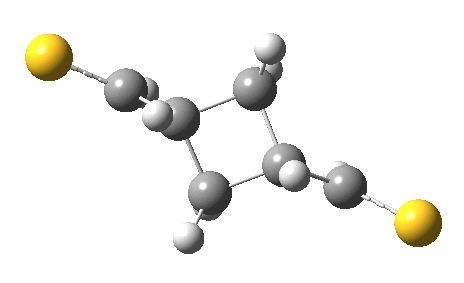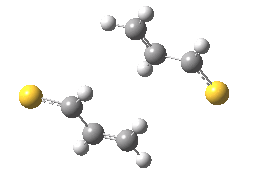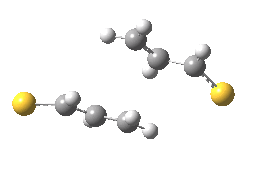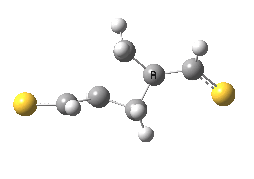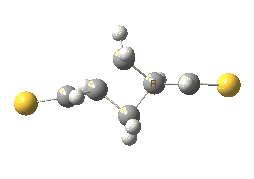The previous post showed how the 2+2 cycloaddition of an alkene could occur by a sort of sideways insinuation of the bonds. I have also shown how the same reaction can occur with a dramatic rotation of one of the double bonds. This post compares the two moves side by side.
| 0.0 | 5.3 kcal/mol |
As is sometimes the case in real life, the forbidden option has the lower activation barrier!
Author
-
Henry Rzepa is Emeritus Professor of Computational Chemistry at Imperial College London.
View all posts
Share this:
Related
Tags: pericyclic, Tutorial material
This entry was posted on Thursday, December 15th, 2011 at 5:36 pm and is filed under Interesting chemistry. You can follow any responses to this entry through the RSS 2.0 feed. You can leave a response, or trackback from your own site.