My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down. The barrier to their interconversion in this case is generated by a rotation about the two single bonds connecting the carbonyl group to the rest of the molecule. Introductory chemistry tells us that the barrier for rotation about such single bonds is low (i.e. fast at room temperature). But is that true here?
It looks fairly obvious that the process above must involve twisting about two bonds, presumably the red and blue ones. If that assumption is correct, one can move on to ask whether the transition state involves a synchronous twisting about each of them, or whether the two bonds twist at different rates (asynchronously). As transition states go, it is akin to asking whether the two bonds that form in e.g. a Diels Alder cycloaddition reaction form at the same rate (a concerted synchronous reaction) or at different rates (a concerted asynchronous reaction) or even where one bond is complete before the other has started to form (a non-concerted reaction). At least in that situation, we are pretty sure that the reaction coordinate is appropriately defined by the two C-C bonds. So on to atropisomerism. If you click on the above diagram, you will get an animation of the transition mode for the atropisomeric process in this taxol-like molecule. Look at it carefully and decide for yourself where the rotation is actually taking place.
The next diagram is the IRC (intrinsic reaction coordinate) for the process. Unlike the transition mode which is a harmonic motion, the IRC can be anharmonic. This in turn means the reaction coordinate can evolve, and mutate as the reaction proceeds!
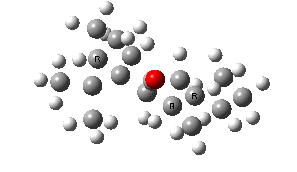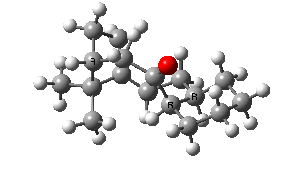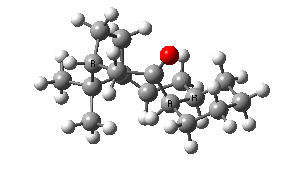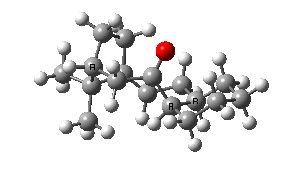
IRC for taxol atropisomerism
- The process occurs in stages. Firstly, the carbonyl group rotates (red and blue bonds)
- This then stops, and the methylene group starts to rotate (green and red bonds). This is the motion that is occurring at the actual transition state.
- The methylene group rotation then slows down and the carbonyl group starts up again (red and blue bonds).
| IRC | Gradients of IRC |
|---|---|
As reactions go therefore, this one is defined by quite a complex reaction coordinate, most definitely not a simple twist about the two most obvious single bonds.
Tags: animation, conformational analysis, Taxol, Tutorial material
[…] be described by the chemical name atropisomerism, a topic I dealt with earlier with the example of Taxol and which is a conformational […]
[…] The IRC reveals all. Put simply, the initial Ru complex has a trigonal bipyramidal geometry. Such a shape has no free ligand site large enough to accommodate an incoming alkene. A free site can be however generated by changing the metal coordination to square pyramidal. So the initial approach of an alkene plays that role, by effectively repelling the Cl and Ru=CH2 ligands into the square pyramidal geometry. This process by the way is not dissimilar[1] to pseudorotation in PCl5. No C-C bond formation can happen whilst this geometrical reorganisation takes place (another example of high barriers induced purely by changes in bond angles is atropisomerism in taxol). […]
[…] The transition state with C2 symmetry is in fact 10.1 kcal/mol lower in free energy than the one with Cs symmetry. So the arrows follow the aromaticity (or vice versa), and this determines the stereochemistry (axis or plane of symmetry) and ultimately the nature of the product of each reaction. Are these annulenes indeed different? Shown below are the final outcomes of following an IRC (intrinsic reaction coordinate) from the transition state of the red and the blue reaction downhill to the [12]-annulenes. Not only is the outcome valence bond isomers, but they are also atropisomers. […]
[…] of this transition state, I illustrate another isomerism that 6 can undertake; a low-barrier atropisomerism to form 9, followed by another reaction with a relatively low barrier, 9 ↠ 7 to give the […]