I wrote earlier about the strangely close contact between two hydrogen atoms in cis-butene. The topology of the electron density showed characteristics of a bond, but is it a consensual union? The two hydrogens approach closer than their van der Waals radii would suggest is normal, so something is happening, but that something need not be what chemists might choose to call a “bond“. An NCI (non-covalent analysis) hinted that any stability due to the electron topologic characteristics of a bond (the BCP) might be more than offset by the repulsive nature of the adjacent ring critical point (RCP). Here I offer an alternative explanation for why the two hydrogens approach so closely.
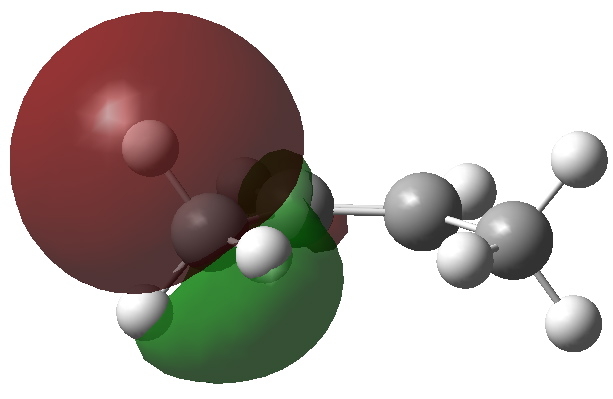 One of four C-H NBO donor orbitals. Click for 3D |
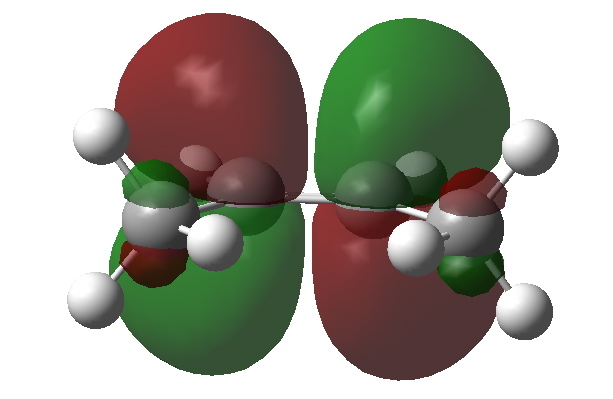 The empty C=C π* orbital. Click for 3D to show both orbitals superimposed. |
We need to try to “concentrate” or “focus” the effect into the bonds of the molecule, and a good way of doing this is to calculate the NBO (natural bond orbitals). The first we focus on is localised onto one (of four) C-H bonds of the methyl group; the other is the anti bonding π* orbital of the alkene. NBO theory allows us to calculate how these two orbitals perturb each other, in the sense of the occupied orbital donating to the empty orbital. This energy is known as E(2), and for any of the four (equivalent) interactions above, it is computed at 5.17 kcal/mol. If you look closely at the orbitals, the C-H bond is leaning away from the centre, but so is the π* acceptor orbital (that is the nature of anti bonding orbitals). These characteristics improve the overlap of the orbitals, and hence tend to increase the value of E(2).
What about an alternative conformation of cis-butene in which the close contact of the H…H atoms is removed by rotation? Well, the C-H NBOs now rotate in, but the anti bonding π* orbital still tilts out. The overlap between them is no longer quite so good, and indeed the E(2) energy decreases to 4.43 kcal/mol.
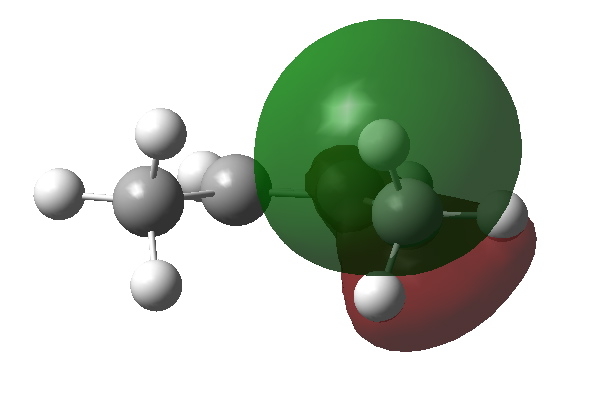 Donor C-H bond in rotated isomer of cis-butene. Click for 3D |
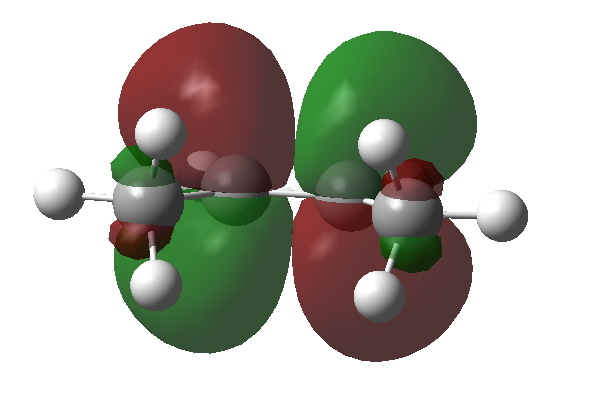 NBO C=C p* orbital in rotated isomer of cis-butene. Click for 3D |
How many more orbitals should be considered? Well, the NBO technique in effect concentrates these effects into a relatively small number of orbitals (those separated by the smallest energy gap). We can also add in the four interactions between the bonding π orbital and the anti-bonding C-H* NBO. The totals for the first conformation come to 34.08 and for the second 30.44.
So we can conclude by observing that cis-butene makes a sacrifice for its greater good. Rotating the methyl groups means that the overlap of four C-H bonds with the alkene is optimised, but an undesired side effect is to induce two hydrogens to get close to each other. They would not normally be happy doing so, but the gain from the first effect is greater than the loss from the second. Whilst they may be close, chemists would prefer not to call the H-H approach a bond, even though the topology of the electron density might say it is.
By the way, this is my 150th post. I had little idea when I started that I might reach this milestone.
Tags: conformational analysis, energy, energy decreases, smallest energy gap, Tutorial material
[…] Well, not quite. A chemist would still ask why structure (a) is preferred over structure (b), if the wider H…H region is not deemed attractive (I call it a region rather than a bond in a probably futile attempt to avoid controversy). Actually, the answer might be in how the two methyl groups each interact with the other part of the molecule, the alkene, and how that might depend on their orientation with respect to the alkene. But that analysis is for another post! […]
[…] Henry Rzepa Chemistry with a twist « Are close H…H contacts bonds? The denouement! […]
[…] and moreover the active space orbitals are probably not identical either (I demonstrated in another post how the orbitals of the alkene interact with those of the methyl groups, and its quite likely that […]
[…] I have pointed out elsewhere that the existence of such a topological feature does not necessarily coincide with what we think […]
[…] the scaffold results in a greater overlap of the C=C π and H-O σ* NBOs. Recollect however the H…H distance in cis-butene, a proximity that was enforced by other effects and which I argued was NOT a chemical […]
[…] In the range IRC ± (9 – 15), unexpected features appear (hidden intermediates if you check this post). A whole plethora of them. This is the conformational region where the methyl flags start waving (and no bonds are formed or broken). If you watch the animation above very carefully, you will note that the methyl groups start rotating at the start and at the end of the migration, at a stage when the ring has an allyl cation. This delocalised cation has a different impact upon the conformation of the methyl groups from that of the transition state, where the charge now resides largely on the migrating carbon, and the ring now has just a neutral butadiene. This latter imparts a different conformational preference upon the methyl groups. You can see an orbital analysis of these effects at this post. […]
[…] molecules are shown above; in effect propene 1 and butene 2. The latter was in fact the topic of another post, in which I attempted to show that the close H…H contact in cis-butene (2.1Å) was in effect […]
[…] from two C-H bonds (R=H above) into the π*C=O NBO orbital (in the manner that was used to explain the cis-orientation of the two methyl groups in […]
[…] the X-ray structure[1] that the H…H distance was in the region of 1.50Å. It’s that cis-butene all over again! So is that H…H region a bond? Is it attractive or repulsive? Go read […]