To (mis)quote Oscar Wilde again, ““To lose one methyl group may be regarded as a misfortune; to lose both looks like carelessness.” Here, I refer to the (past) tendency of molecular modellers to simplify molecular structures. Thus in 1977, quantum molecular modelling, even at the semi-empirical level, was beset by lost groups. One of my early efforts (DOI: 10.1021/ja00465a005) was selected for study because it had nothing left to lose; the mass spectrometric fragmentation of the radical cations of methane and ethane. Methyl, phenyl and other “large” groups were routinely replaced by hydrogen in order to enable the study. Cations indeed were always of interest to modellers; the relative lack of electrons almost always meant unusual or interesting structures and reactions (including this controversial species, DOI: 10.1021/ja00444a012). Inured to such functional loss, we modellers forgot that (unless in a mass spectrometer), cations have to have a counter anion. Here I explore one example of the model being complete(d).
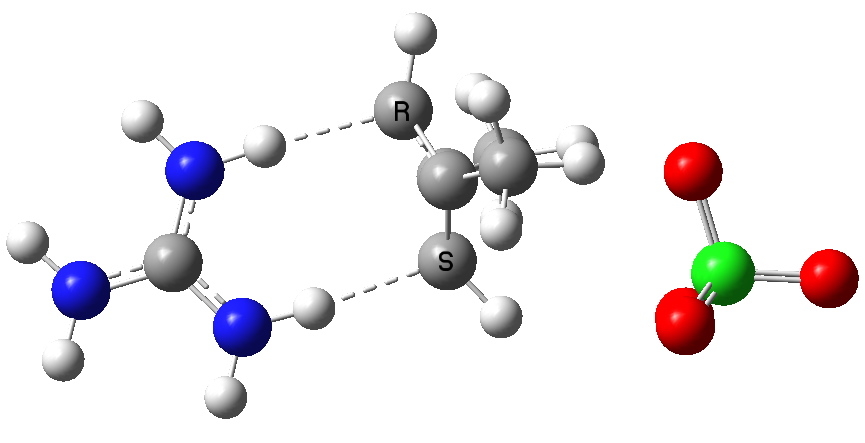
The ion-pair complex of cyclobutadiene.
What are the implications to a modeller of adding counter ions? Well, when you start doing such calculations, you find that the practical matter of optimising the geometry is not quite as straightforward as it is found to be for what I would call covalently bonded systems. These latter have pretty predictable geometries, and these geometries are pretty rigid. Ion-pairs on the other hand are less predictable. Note for example in the above diagram that the perchlorate counterion sits to one side of the molecule, and is not symmetrical. The potential energy surface can be very flat indeed, which means that locating the optimal geometry can be quite a struggle. And unlike a covalent structure, where once the location of the covalent bonds is decided, there is little further ambiguity, ion-pairs may have many different possible relative orientations. Thus the above one may not be unique!
But the last word to this post should be: do not forget counter ions if you a looking at ionic species, and always strive to be complete!
Tags: bonded systems, energy difference drops, free energy, model, Oscar, potential energy surface, Steve Bachrach, using perchlorate
So what is your recipe to solve problems with unsymmetrical multiatomic ion pairs? One has to try as many initial configurations as he can make up and also include various orientations of ions relative to each other? What about the initial separation between ions in the starting configuration?
P.C. I’m talking about the situations where it is unaffordable to run a complete MD/MC simulation.
I do not have a comprehensive recipe, and its clearly a need for someone to develop methodologies in this area.
What I did have to do to optimise the ion-pair in the (relatively flat) potential is to calculate 2nd derivatives at EACH step of the optimisation! Wow!
[…] the principle of completeness, it is important to include a counterion. And because the colour mauve is recorded in methanol […]
[…] I had shaved off the four benzo groups (blue) in the time honoured computational tradition of clearing away distractions. Unfortunately, it became clear as the story unfolded that the benzo groups had a distractingly […]
[…] with modelling a cation without a counter-ion, I added one. I noted that the cyclobutadiene+ ion pair was more stable in this more complete […]
[…] thoughts in fact followed a point I have been making here recently, the principle that modelling a complete system is probably better than a partial one. Now, if you look at Figure 1 of the Singleton […]
[…] this time is eliminated by forming an allyl cation on the ring. I have described this mode in another post, commenting on the effect when a guanidinium cation interacts with […]