It is not often that an article on the topic of illusion and deception makes it into a chemical journal. Such is addressed (DOI: 10.1002/anie.201102210) in no less an eminent journal than Angew Chemie. The illusion (or deception if you will) actually goes to the heart of how we represent three-dimensional molecules in two dimensions, and the meanings that may be subverted by doing so. A it happens, it is also a recurring theme of this particular blog, which is the need to present chemistry with data for all three dimensions fully intact (hence the Click for 3D captions which often appear profusely here).
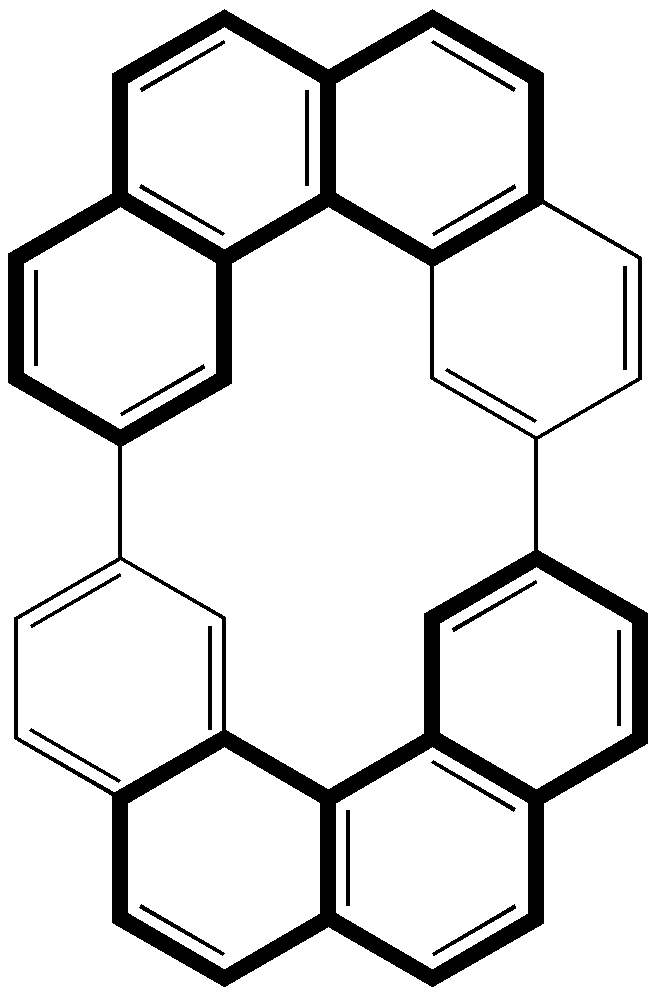
Molecular Penrose stair. Click for 3D.
What might be of (chemical) interest about this molecule, other than its illusory aspects? Well, could you for example work out, given ONLY the representation above, that the molecule has D2 symmetry, and is therefore chiral? I wondered if it might also be Möbius (with perhaps two half twists), although in fact the π-system in this case has a linking number Lk of zero (not 2). It also has some interesting rather close H…H contacts in the middle, and the inner periphery appears to be a 14-annulene and the outer 26, both conforming to the Huckel 4n+2 rule. Despite this the central C-C bond is actually quite long, and the conjugation is hence significantly interrupted. There is more chemistry in the original article.
But I want to close here on the point that to overcome the deception and illusion, you need to get the three dimensional data in chemistry. You can do this by clicking above. Or, by going to the original article and striving to do so there. I think you will find the latter route the greater challenge! Then, ask yourself why an eminent journal, in publishing an article on the topic of deception and illusion, makes it so relatively difficult to overcome that illusion. Certainly more difficult than I hope it proves to be on this blog!
Tags: chemical, chemical journal, chemist, M. C. Escher, Tutorial material
I think the origin of this illusion is simply a problem of perspective.
The helicene can be thought of as having an inner rim of carbon atoms, with a radius similar to that of benzene, and an outer rim of carbon atoms, with a radius similar to that of 18-annulene. In the case of this molecule, if you take the two “linking” bonds to be in the plane of the page (or screen) rather than the faces, then the inner rim descends clockwise but, crucially, the outer rim ascends clockwise.
I think the problem is that your line drawing (and the schematic from the paper) suggests that both of these carbon “rims” are descending clockwise. This is not the case, hence why drawing it in this way suggests an impossible structure.
I think that there is a better way of drawing this molecule in 2D which overcomes this problem, enabling it to be more easily mentally visualised, but I can’t work out how to attach the picture.
[…] start from the top diagram, which is a schematic 2D representation of the molecule. As you can read in this post, such representations can often be illusory, or even contradictory. One is indeed lucky if they are […]