This molecule is not leaving me in peace. It and I first met in 1990 (DO: 10.1039/C39910000765), when we spotted the two unusual π-facial bonds formed when it forms a loose dimer. The next step was to use QTAIM to formalise this interaction, and this led to spotting a second one missed the first time round (labelled 2 in that post). Then a method known as NCI was tried, which revealed an H…H interaction, labelled ? in that post! Here I discuss the origins of the ?
What sparked this re-visitation? Firstly, in this post, a CH…O interaction in Z-DNA was identified using NCI, and its origins probed using NBO E2 perturbation energies, which revealed that the C-H bond was antiperiplanar to a C-O bond (effects 2 and 3 in that post), that could have the effect of acidifying the H, and making it more prone to hydrogen bond to the lone pair of an oxygen. Inspired by this, I worked out how to display NCI colour codes surfaces here on this blog using Jmol (previously, VMD had been used, which cannot be embedded in a blog).
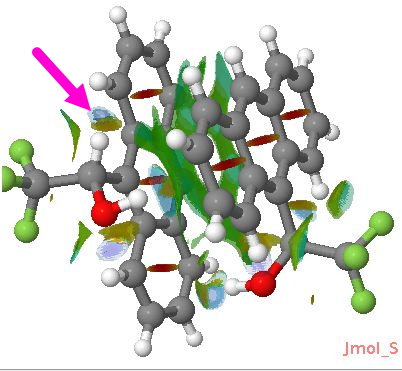
NCI Surface for the Pirkle reagent. Click for 3D
You may notice from the above other blue regions. Click on the diagram, and go explore them. The history of this molecule is such that it is bound to hold more surprises!
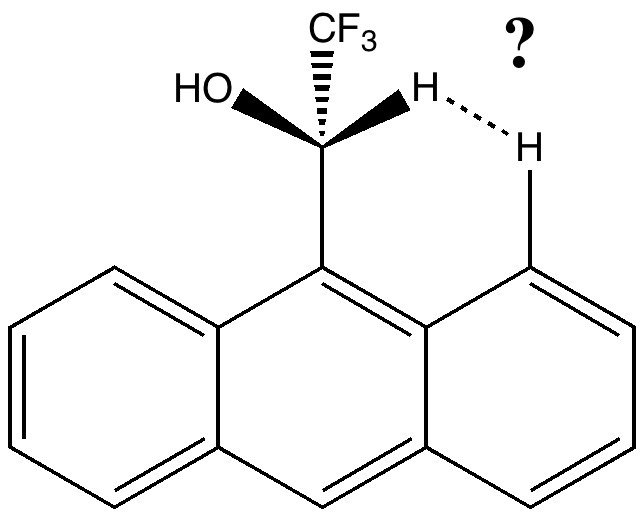
[…] Pirkle reagent is a 9-anthranyl derivative (X=OH, Y=CF3). The previous post on the topic had highlighted DIST1, the separation of the two hydrogen atoms shown below. The next […]