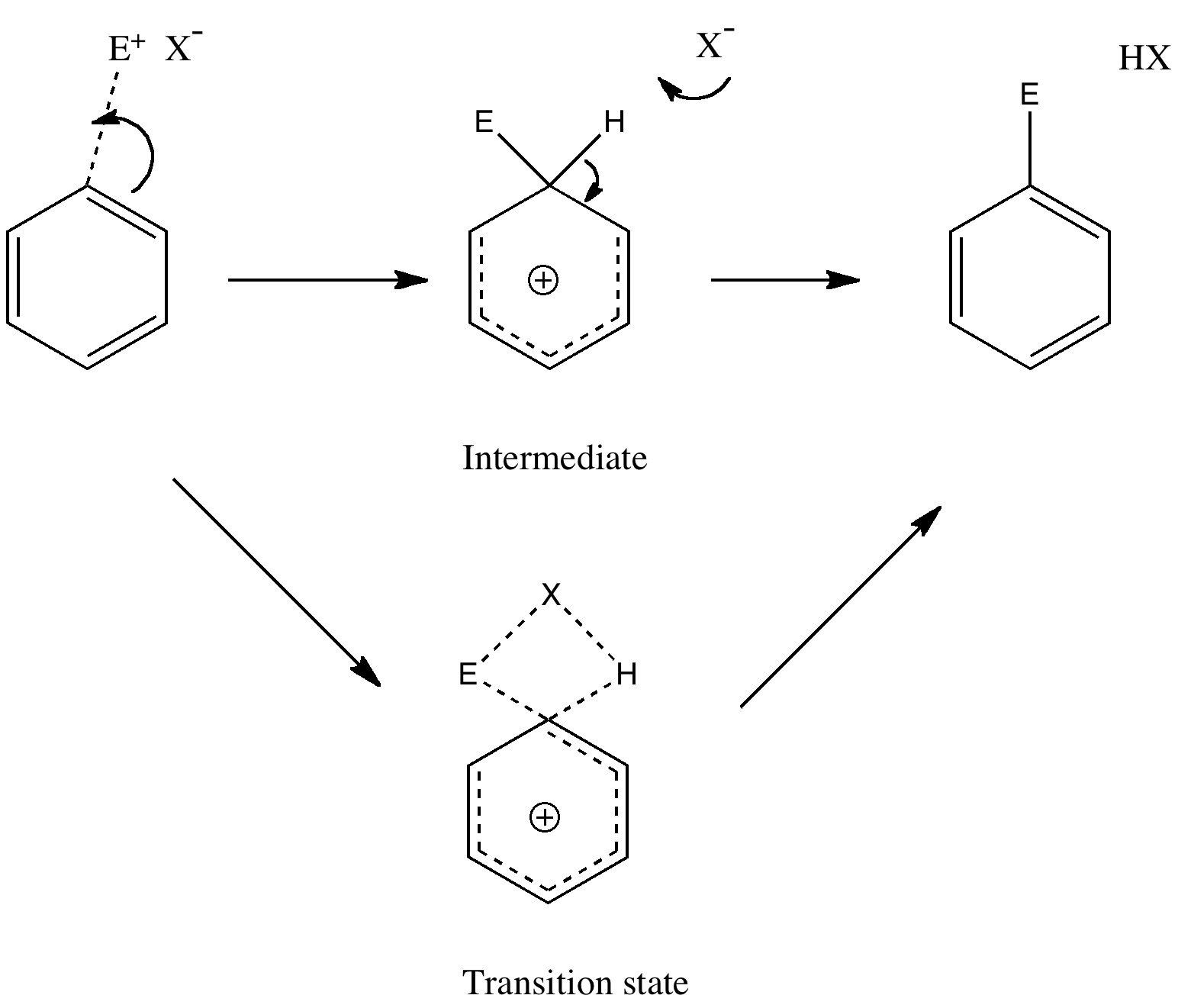
Unravelling reaction mechanisms is thought to be a 20th century phenomenon, coincident more or less with the development of electronic theories of chemistry. Hence electronic arrow pushing as a term. But here I argue that the true origin of this immensely powerful technique in chemistry goes back to the 19th century. In 1890, Henry Armstrong proposed what amounts to close to the modern mechanism for the process we now know as aromatic electrophilic substitution [1]. Beyond doubt, he invented what is now known as the Wheland Intermediate (about 50 years before Wheland wrote about it, and hence I argue here it should really be called the Armstrong/Wheland intermediate). This is illustrated (in modern style) along the top row of the diagram.
In 1887, Armstrong had tabulated the well known ortho/meta/para directing properties of substituents already on the ring towards this reaction[2]. He even offered an explanation, which is not entirely wrong, given that in 1890, the electron had not yet been discovered! That did not stop Armstrong, who invented an entity he called the affinity for the purpose of developing his theories (in this theory, benzene had an inner circle of six affinities, which had a tendency to resist disruption). Armstrong’s description of the properties of the affinity matches that of the (yet to be discovered) electron very closely! But that is enough of history. The mechanism shown above emerged in its present representation (and naming) during the heyday of physical organic chemistry between 1926 – 1940, and of course is an absolute staple of all text books on organic chemistry. But, sacrilege, is it correct? Could what is referred to as an intermediate instead be a transition state? (shown in the bottom pathway of the scheme).
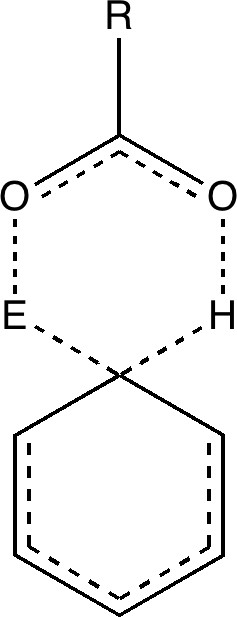
Consider instead the following, in which X is replaced by an acetic acid motif;
The two steps, a bond formation between the benzene and E, and the proton abstraction from the benzene by X, are now synchronized into a single step, and the intermediate is now transformed into a transition state. Time to put this theory to the test. X is going to be made trifluoroacetate (R=CF3) and we are going to test it with E= NO+ and F+ (yes, trifluoroacetyl hypofluorite is a known chemical, and it really does fluorinate1 aromatic compounds at -78C). Firstly, E= NO+. A B3LYP/6-311G(d,p) calculation[3] run in a solvent simulated as dichloromethane, reveals the mid point to indeed be a transition state and NOT an intermediate![4].

Wheland as a transition state. Click image for animation
There is one crucial aspect to this transition state that Armstrong himself made a point of. In the Wheland intermediate proper, the aromaticity of the benzene ring must be disrupted. As a transition state, it need not be (at least not completely). Thus the two bonds labeled as a have calculated lengths of ~1.415Å, only slightly longer than the aromatic length, and certainly not single bonds as implied by the Wheland intermediate! Notice also the significant motion by the hydrogen, which implies the reaction would be subject to a kinetic isotope effect (this would normally be interpreted in terms of the second stage of the stepwise reaction shown along the top a being rate limiting, but this result shows this need not be so). Thus, if the structure is favourable, this veritable old mechanism can be redesigned to give a new, 21st century look to a 19th century staple! By the way, the free energy of activation for this reaction is calculated as ~22 kcal/mol, a perfectly viable thermal reaction. No doubt, by suitable design of the group X, this might be reduced.
Now on to E=F+[5]. This looks a little different. F+ is now a much more voracious electrophile than the nitrosonium cation, and it therefore jumps ahead of the second mechanistic step, with no motion of the hydrogen being involved at this stage (one might also imagine making X a better base to swing things the other way).
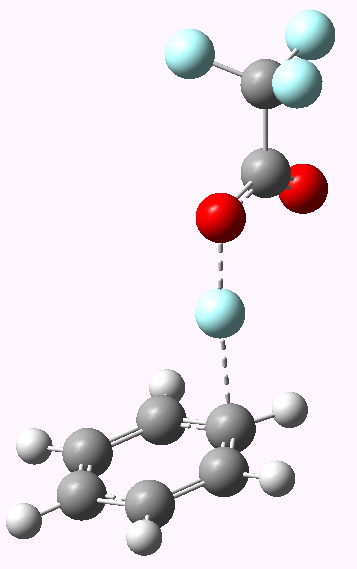 Transition state E=F+ leading to Wheland Intermediate. Click for 3D model. |
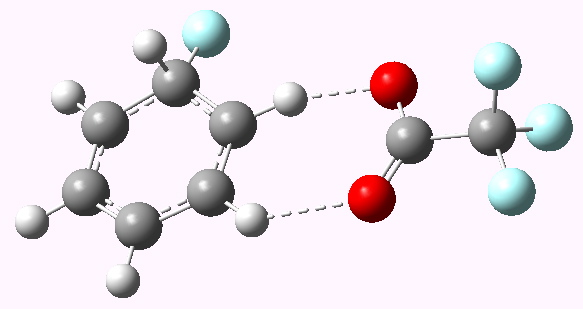 Genuine Wheland intermediate for E=F+ Click for 3D model |
Now a full blown Armstrong/Wheland intermediate does indeed form (10042/to-5174); an intimate ion pair if you will, even in the relatively non polar dichloromethane as modelled solvent. The structure (shown above) is rather unexpected. This reaction has ΔG† of ~5 kcal/mol, which is significantly lower than for the E=NO+ system.
Chemistry is full of surprises, and it is always a wonder how a slightly different take on even the oldest of reactions can reveal something new.
Reference.
<
p>1. Umemoto, T.; Mukono, T.. 1-Acylamido-2-fluoro-4-acylbenzenes. Jpn. Kokai Tokkyo Koho (1986), Patent number JP61246156.
References
- "Proceedings of the Chemical Society, Vol. 6, No. 85", Proceedings of the Chemical Society (London), vol. 6, pp. 95, 1890. https://doi.org/10.1039/pl8900600095
- H.E. Armstrong, "XXVIII.—An explanation of the laws which govern substitution in the case of benzenoid compounds", J. Chem. Soc., Trans., vol. 51, pp. 258-268, 1887. https://doi.org/10.1039/ct8875100258
- S.R. Gwaltney, S.V. Rosokha, M. Head-Gordon, and J.K. Kochi, "Charge-Transfer Mechanism for Electrophilic Aromatic Nitration and Nitrosation via the Convergence of (ab Initio) Molecular-Orbital and Marcus−Hush Theories with Experiments", Journal of the American Chemical Society, vol. 125, pp. 3273-3283, 2003. https://doi.org/10.1021/ja021152s
Tags: acetic acid, animation, aromaticity, chemical, free energy, Henry Armstrong, Historical, Interesting chemistry, Wheland intermediate
[…] of a university tutor: (curly) arrow pushing Curly arrows are something most students of chemistry meet fairly early on. They rapidly become hard-wired into […]
[…] future. Some of the most ancient (i.e. 19th century) known reactions can be considered part of a chemical mythology; perhaps it is time for a Janus-like look into their […]
I really enjoyed reading this piece.
Keep ’em coming!!
-Sean
This is very interesting…
What would happen if we put some bulky groups on the aromatic ring? Will this TS still be favoured?
Michael
[…] to infer where the charge is. The latter two emerge as different resonance forms of what we call Wheland intermediates. The former is the silicon equivalent of a tertiary carbocation. Quantum mechanics now tells us […]
[…] me set the scene on how this might be done. Although aromatic electrophilic substitutions are the grand-daddy of all mechanisms, they present some computational challenges. An electrophile is needed, and this […]
[…] rate of a reaction) more closely resembles the (initial) product of this reaction, the so-called Wheland intermediate rather than indole itself? I am going to calculate this intermediate in a novel manner; as an […]