About 18 months ago, there was much discussion on this blog about a system reported by Bob Pascal and co-workers containing a short H…H contact of ~1.5Å[1]. In this system, the hydrogens were both attached to Si as Si-H…H-Si and compressed together by rings. Now a new report[2] and commented upon by Steve Bachrach, claims a similar distance for hydrogens attached to carbon, i.e. C-H…H-C, but without the ring compression.
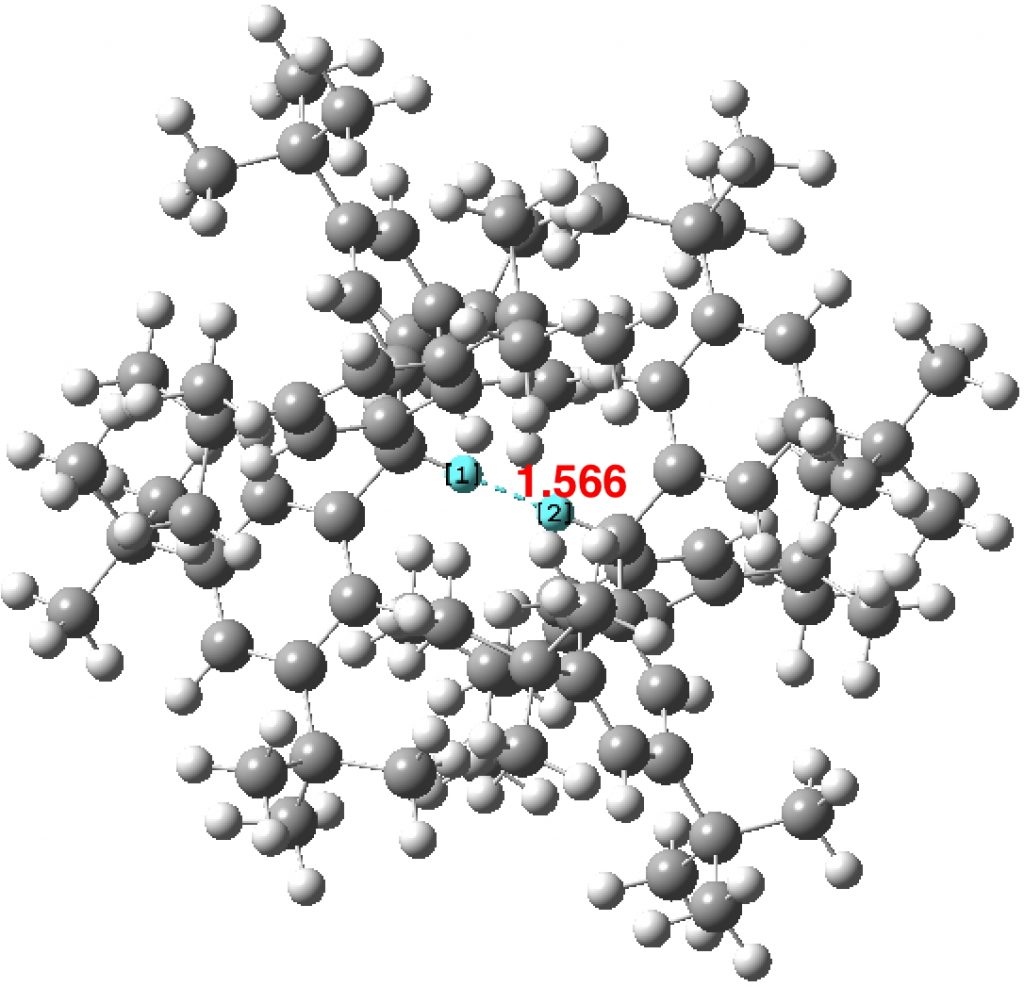
This new example is the structure of an C3-symmetric all-meta tBu-triphenylmethane R-H…H-R dimer determined by neutron diffraction (DOI: 10.5517/ccdc.csd.cc1nc1bd) and the close interaction is achieved purely by attractions due to dispersion forces accumulating in the remainder of the molecules. This study also reports a diverse set of computed properties for this new system, but one property reported as part of the previous discussion was not presented, the 1JH-H coupling constant. I have computed it here in the hope that it might be possible to measure by some means, perhaps in the solid state?
The chemical shift of the R3CH proton is measured as a singlet‡ at ~7.35 ppm (in deuterated benzene, Figure S6, SI).† 
The value calculated using B3LYP/Def2-TZVPP (gas phase) is 7.39 and 7.69 ppm (averaged to 7.54 for a rapidly exchanging environment). The 1J coupling is calculated as 4.3 Hz at the B3LYP/Def2-TZVPP level, DOI: 10.14469/hpc/2699. The designation 1J is normally taken as a 1-bond pathway for the coupling. In this example, the designation of the H-H region as a “bond” is the interesting discussion point!
I end by noting here my observation that although the neutron diffraction study of ammonium tetraphenylborate shows the N-H protons as pointing directly towards the centroid of phenyl groups, the original observation[3] was made that “even at 20 K the ammonium ion performs large amplitude motions which allow the N-H vectors to sample the entire face of the aromatic system”. The equivalent thermal motion for the triphenylmethane system here would have the C-H vectors orbiting around each other in a manner that increases the H-H separation, but which averages out to them pointing directly towards one another? The calculated normal coordinate analysis of this system is not available from the article SI, so the ease of C-C-H bending to achieve such motion is difficult to ascertain. Perhaps trying to detect the 1J coupling might illuminate whether this happens?
Postscript. †Prof Schreiner has indicated that that the methine assignment is 5.79 ppm (b below) and not 7.35 as marked with a diamond in the S6 figure caption (a below). This is of course measured in d6-benzene solution and applies to the monomer, not presumably the dimer. The calculated value of 7.54 ppm as reported above applies specifically to the dimer, which suggests a significant shift of ~2ppm upon dimer formation. It would be interesting to verify this prediction via a solid-state measurement.
‡Measuring coupling would require an asymmetric environment to differentiate the two chemical shifts of the interacting hydrogens. Although the C3 symmetry of the crystal structure could provide such an environment, it is observed to be fluxional in solution, which equalises the two chemical shifts on the NMR time scale. Two non-equivalent protons exhibiting only mutual couplings manifest as an AB-type double doublet of peaks in the NMR spectrum. As the difference in chemical shift between the two nuclei (in units of Hz) approaches in magnitude the value of the coupling constant between them (also in Hz), the AB quartet becomes increasingly second-order in appearance. This means that the intensities of the two outer peaks starts to decrease and the two inner peak intensities increase. When the chemical shift difference between them reaches zero, the intensity of the two outer peaks also becomes zero and the two inner peaks superimpose to become a single peak. This means that the coupling constant cannot be measured from the splitting of the peaks (which has vanished). It does not mean of course that the coupling itself has vanished; it merely no longer manifests in the spectrum.
References
- J. Zong, J.T. Mague, and R.A. Pascal, "Exceptional Steric Congestion in an <i>in</i>,<i>in</i>-Bis(hydrosilane)", Journal of the American Chemical Society, vol. 135, pp. 13235-13237, 2013. https://doi.org/10.1021/ja407398w
- S. Rösel, H. Quanz, C. Logemann, J. Becker, E. Mossou, L. Cañadillas-Delgado, E. Caldeweyher, S. Grimme, and P.R. Schreiner, "London Dispersion Enables the Shortest Intermolecular Hydrocarbon H···H Contact", Journal of the American Chemical Society, vol. 139, pp. 7428-7431, 2017. https://doi.org/10.1021/jacs.7b01879
- T. Steiner, and S.A. Mason, "Short N<sup>+</sup>—H...Ph hydrogen bonds in ammonium tetraphenylborate characterized by neutron diffraction", Acta Crystallographica Section B Structural Science, vol. 56, pp. 254-260, 2000. https://doi.org/10.1107/s0108768199012318
Tags: 10.1021, Blog, chemical shift, chemical shift difference, chemical shifts, gas phase, Oxygen, Steve Bachrach
\”This means that the coupling constant cannot be measured from the splitting of the peaks (which has vanished). It does not mean of course that the coupling itself has vanished; it merely no longer manifests in the spectrum.\”
Perhaps, this coupling constant may be measured from the splitting of 13C satellites of the peak if the reaction Ar3CH—HCAr3 = 2 Ar3CH is slow sufficiently.
Yes, that might work. But although the kinetics of the dissociation process have not been measured, I suspect that they will be very fast!
Bob Pascal has recently reported (DOI: 10.1002/anie.201712304) an in,in-cyclophane with two
inwardly directed methine groups, the hydrogen-hydrogen non-bonded contact
distance being ~1.50-1.53 Å, a little bit shorter than the system above. Significantly in this new system, through-space H-H spin coupling of 2Hz was observed, thus confirming the close approach. The 3D coordinates can be viewed at DOI 10.5517/ccdc.csd.cc1q9jbw.