It is not only the non-classical norbornyl cation that has proved controversial in the past. A colleague mentioned at lunch (thanks Paul!) that tri-coordinate group 14 cations such as R3Si+ have also had an interesting history.[1] Here I take a brief look at some of these systems.
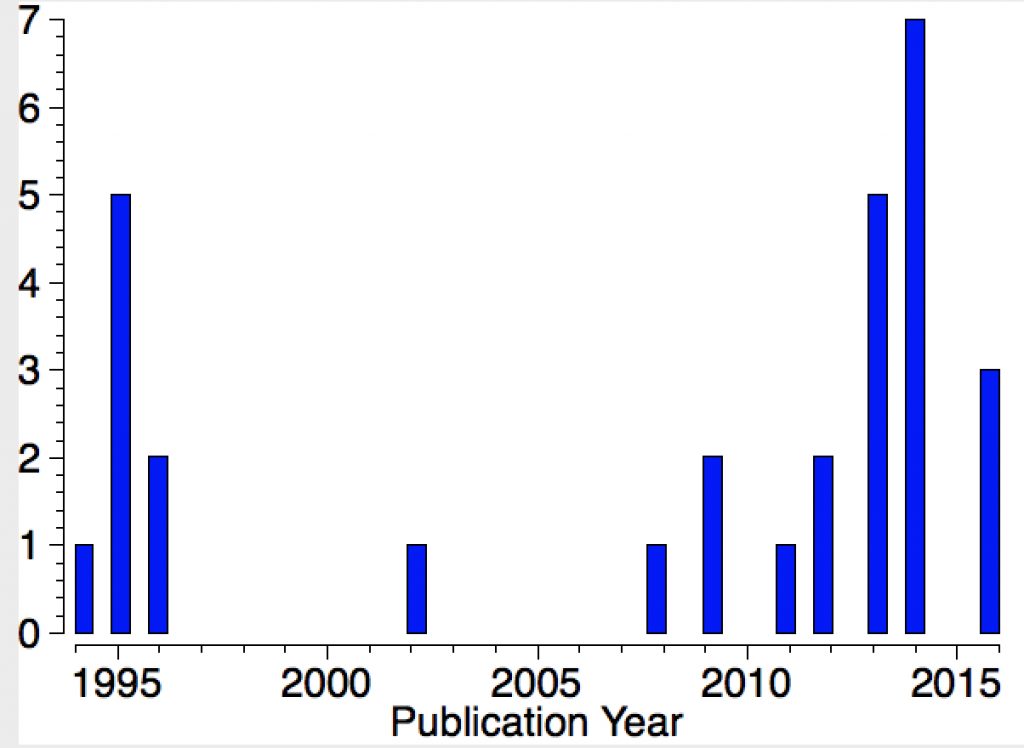
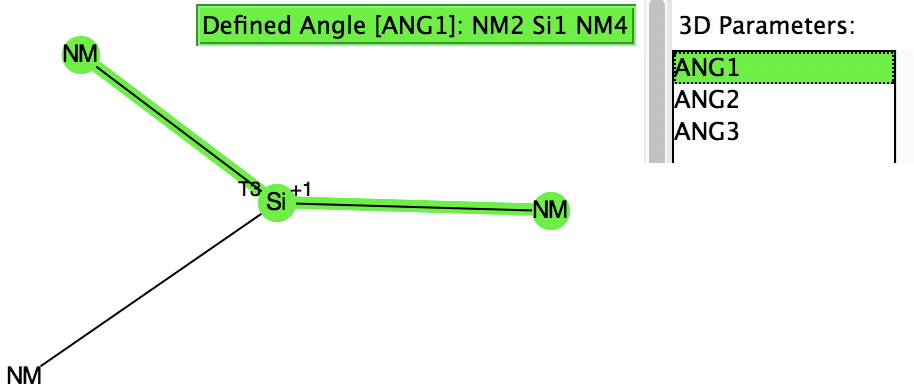
Their initial characterisations, as with the carbon analogues, was by 29Si NMR. The first (of around 25) crystal structures appeared in 1994 (below) and they continue to fascinate to this day. I decided to focus on searching the Cambridge structure database (CSD), using the search query shown below (NM = non-metal). For a planar system the three angles subtended at the Si would of course total to 360°.
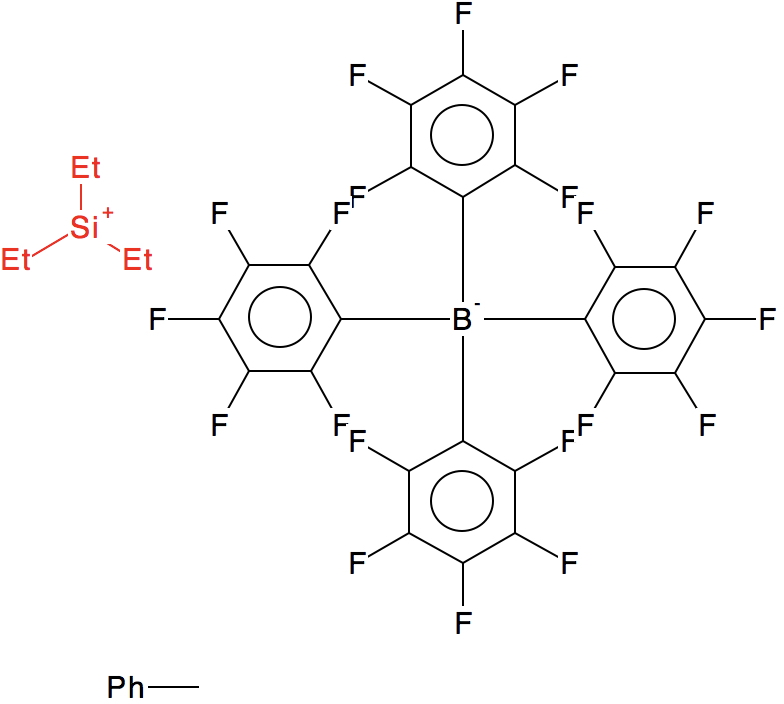
The first such structure, published in 1994[2] is shown in 2D representation below
However, the three angles subtended at the Si are 113, 115 and 114°. Could it be that these types of cation are not planar but pyramidal (a ωB97XD/Def2-TZVPP calculation of SiH3+ certainly gives it as planar). Below is a plot of the three angles:
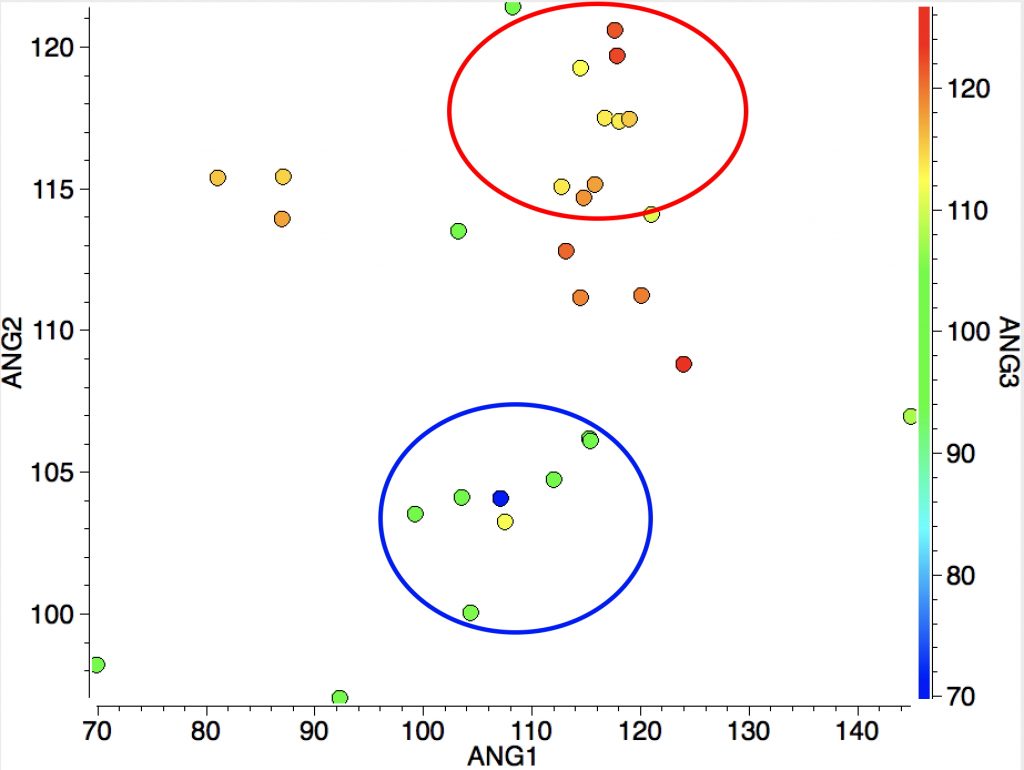
Ringed in red are two systems where all three angles are ~120° (the ones with red dots). The blue circle contains examples where all three angles are <110°. So I took a closer look at the first of these published[2] and known by the code HAGCIB10 (angles of 113, 115 and 114°). The Si appears to be connected to a toluene present in the crystals via an Si-C bond (blue arrow). If correct, that would account for the angles around Si being <120° and indeed closer to tetrahedral, but it would also mean that the species was actually an arenium cation, otherwise known as a “Wheland intermediate”. That extra bond means that it is not a tri-coordinate silicon, but a four-coordinate silicon and that perhaps the indexing in the CSD needs correcting (as was done here).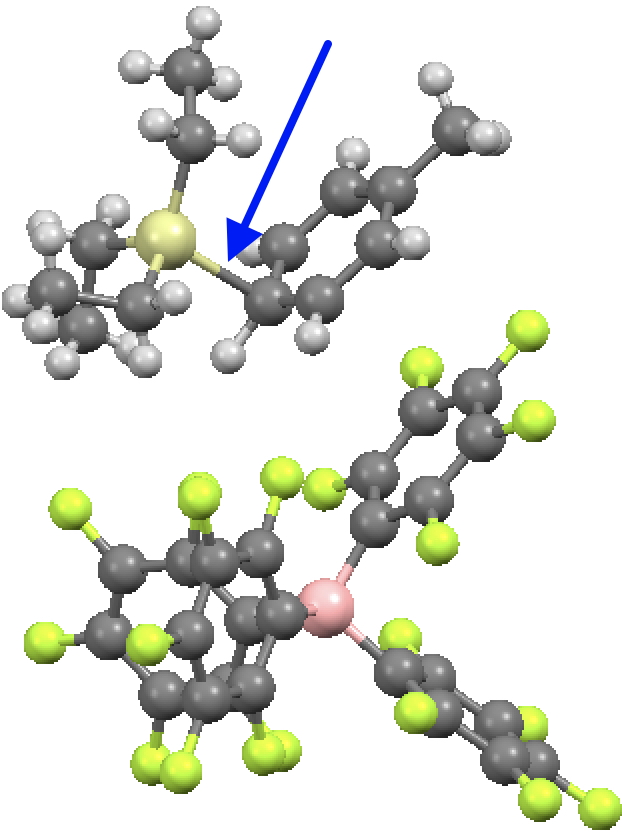
Looking further, quite a few of the 25 examples contain so-called “N-heterocyclic carbene” ligands, as below (DOI for 3D model: 10.5517/CC12FWM0[3]).
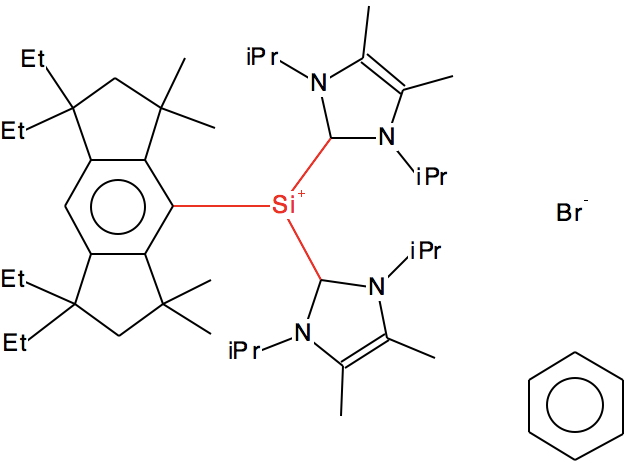
Again one might question the location of the formal +ve charge. Perhaps it might instead reside on the nitrogen as per below, in which case we again do not have a true tri-coordinate silicon cation for systems with such ligands.
This cannot be the whole story, although I would note that Si=C bonds can contain pyramidalised Si. The bonding clearly needs more investigation!
Very probably each of the 25 examples identified by this search as a silylium or silyl cation has its own story to tell. But in unravelling these stories, one should always perhaps take the 2D representations shown in both the CSD and the original publications with a pinch of salt until other possibly better representations such as the one above are excluded.
References
- J.B. Lambert, Y. Zhao, H. Wu, W.C. Tse, and B. Kuhlmann, "The Allyl Leaving Group Approach to Tricoordinate Silyl, Germyl, and Stannyl Cations", Journal of the American Chemical Society, vol. 121, pp. 5001-5008, 1999. https://doi.org/10.1021/ja990389u
- J.B. Lambert, S. Zhang, and S.M. Ciro, "Silyl Cations in the Solid and in Solution", Organometallics, vol. 13, pp. 2430-2443, 1994. https://doi.org/10.1021/om00018a041
- T. Agou, N. Hayakawa, T. Sasamori, T. Matsuo, D. Hashizume, and N. Tokitoh, "Reactions of Diaryldibromodisilenes with N‐Heterocyclic Carbenes: Formation of Formal Bis‐NHC Adducts of Silyliumylidene Cations", Chemistry – A European Journal, vol. 20, pp. 9246-9249, 2014. https://doi.org/10.1002/chem.201403083
Tags: 2-Norbornyl cation, Carbocations, chemical bonding, Chemistry, metal, Physical organic chemistry, Reactive intermediates, search query, tri-coordinate
FWIW, Hiberty and Shaik noted trialkyl silicon halides as being species showing appreciable charge-shift bonding. They attribute it to the tendency for trialkylsilicon cations to have the positive charge densely concentrated on the silicon atom.
Similarly, I read somewhere that the unusual flexibility of the O-Si-O backbone in PDMS appears to be due to some unique property of Si. Silicon is clearly a unique animal!
Here is the Wiberg bond order analysis calculated (ωB97XD/6-311G(d,p), DOI: 10.14469/hpc/2343) for the molecule in Ref 3 (crystal structure, DOI: 10.5517/CC12FWM0). The three C-Si bond orders are 0.76, 0.73 and 0.76.

The charge on the Si is +0.62, which matches the observation made above.

“The Search for an Isolable Silyl Cation Must Continue”
Prof. Dr. Paul von Ragué Schleyer, Peter Buzek, Dr. Thomas Müller, Prof. Dr. Yitzhak Apeloig, and Prof. Dr. Hans-Ullrich Siehl
Angewandte Chemie International Edition in English
Volume 32, Issue 10, pages 1471–1473, October 1993
Yes, it was this aspect that I alluded to as “controversial”. There were a number of claims to have identified the silyl cation, most prominently by Joseph Lambert. Doubts were cast on these in turn by Paul Schleyer, as the reference above suggests. In fact, Lambert did eventually provide convincing evidence, which as I understand won George Olah over, but not immediately Paul Schleyer (personal communication from my colleague). The dispute between Lambert and Schleyer rumbled on, but eventually the “search for an isolable silyl cation” did end successfully to most people’s satisfaction. In particular Christopher Reed produced a number of convincing crystal structure determinations, all of which appear in the diagram above as entries. I note again the caveat that the angles subtended at the Si are not always 120°.
As per usual the Cambridge people have been wonderfully helpful in looking at the structures. I received a long response, but quote this bit here;
I have looked at the list of 25 structures you mention and have made edits to HAGCIB10 and additionally YEWSIE and GIXVIT (these fall into the bracket of a fresh pair of eyes on a different day, taking a slightly different perspective, helped of course by your blog!).
The structure noted above (HAGCIB10, 10.1021/om00018a041) was the subject of Linus Pauling’s last article published in 1994 (DOI: 10.1126/science.263.5149.983). He points out the bond “missing” in the original representation as having a significant bond order and therefore the system constitutes four-coordinate Si rather than three.
He comments on the C-Si bond length of 2.18 Å as being ~0.24Å longer than the sum of the covalent radii of C and Si. This elongation nowadays might be ascribed to hyperconjugation of the C-Si bond with the aryl ring, thus increasing the aromaticity of the “Wheland intermediate”.
I might add that other structures show similar effects, DOI: 10.5517/CCY5FS9 C-Si = 2.173Å, DOI: 10.5517/CCY5FVC C-Si = 2.183Å, DOI: 10.5517/CCY5FYG C-Si 2.140Å, DOI: 10.5517/CCY5FZH C-Si 2.137Å (all taken from DOI: 10.1021/ja209693a) and DOI: 10.5517/CCXBK0S, Si-C 2.121Å
All these structures are indexed in the CSD database as 4-coordinate SI+, as shown below. Perhaps a better representation might be to have the +ve charge indicated on the arene ring, as it would be for an arenium cation?
Following on from my noting Linus Pauling’s last published article, this DOI: 10.1021/ar00051a600 is also well worth a read. It is a letter to an editor dated 9th April, 1994 and published as is in the journal. He died in August of that year.
I cannot resist pointing to another “controversial” example of bonding, boiled down to “is it a bond or not?” In that example, the original article claimed there was NO bond between a carbon and an oxygen approaching to within 1.6Å and others claiming that this WAS a bond.
The parallel to the silyl cation issue is close. In each case, the identity of the compound does rather hinge on whether that bond is present or not! Furthermore, although the bond at issue was longer than the standard covalent length, good chemical reasons in both examples can be found to explain its lengthening.