The anomeric effect is best known in sugars, occuring in sub-structures such as RO-C-OR. Its origins relate to how the lone pairs on each oxygen atom align with the adjacent C-O bonds. When the alignment is 180°, one oxygen lone pair can donate into the C-O σ* empty orbital and a stabilisation occurs. Here I explore whether crystal structures reflect this effect.
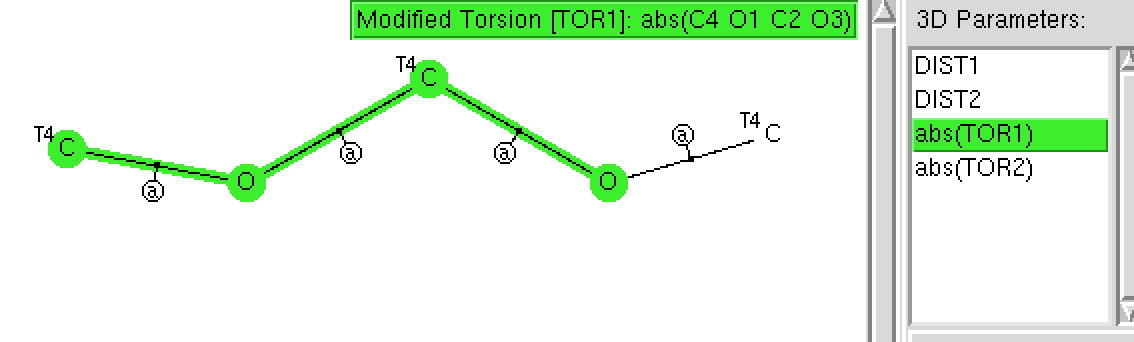
The torsion angles along each O-C bond are specified, along with the two C-O distances. All the bonds are declared acyclic, and the usual R < 5%, no disorder and no errors specified.
- You can see from the plot below that the hotspot occurs when both RO-CO torsions are ~65°. From this we will assume that the two (unseen)‡ lone pairs at any one of the oxygens are distributed approximately tetrahedrally around each oxygen, and if this is true then one of them must by definition be oriented ~ 180° with respect to the same RO-CO bond (the other is therefore oriented -60°). This allows it to be antiperiplanar to the adjacent C-O bond and hence interact with its σ* empty orbital. So the hotspot corresponds to structures where BOTH oxygen atoms have lone pairs which interact with the adjacent O-C anti bond.
- There is a tiny cluster for which both RO-CO torsions are ~180° and hence neither oxygen has an antiperiplanar lone pair.
- Only slightly larger are clusters where one torsion is ~65° and the other ~180°, meaning that only one oxygen has an antiperiplanar lone pair.
- A plot of the two C-O lengths indeed shows an overall hotspot at ~1.40Å for both distances. If the search is filtered to include only torsions in the range 150-180°, the hotspot value increases to 1.415Å for both. If one torsion is restricted to 40-80° and the other to 150-180° the hotspot shows one C-O bond is about 0.012Å shorter than the other.
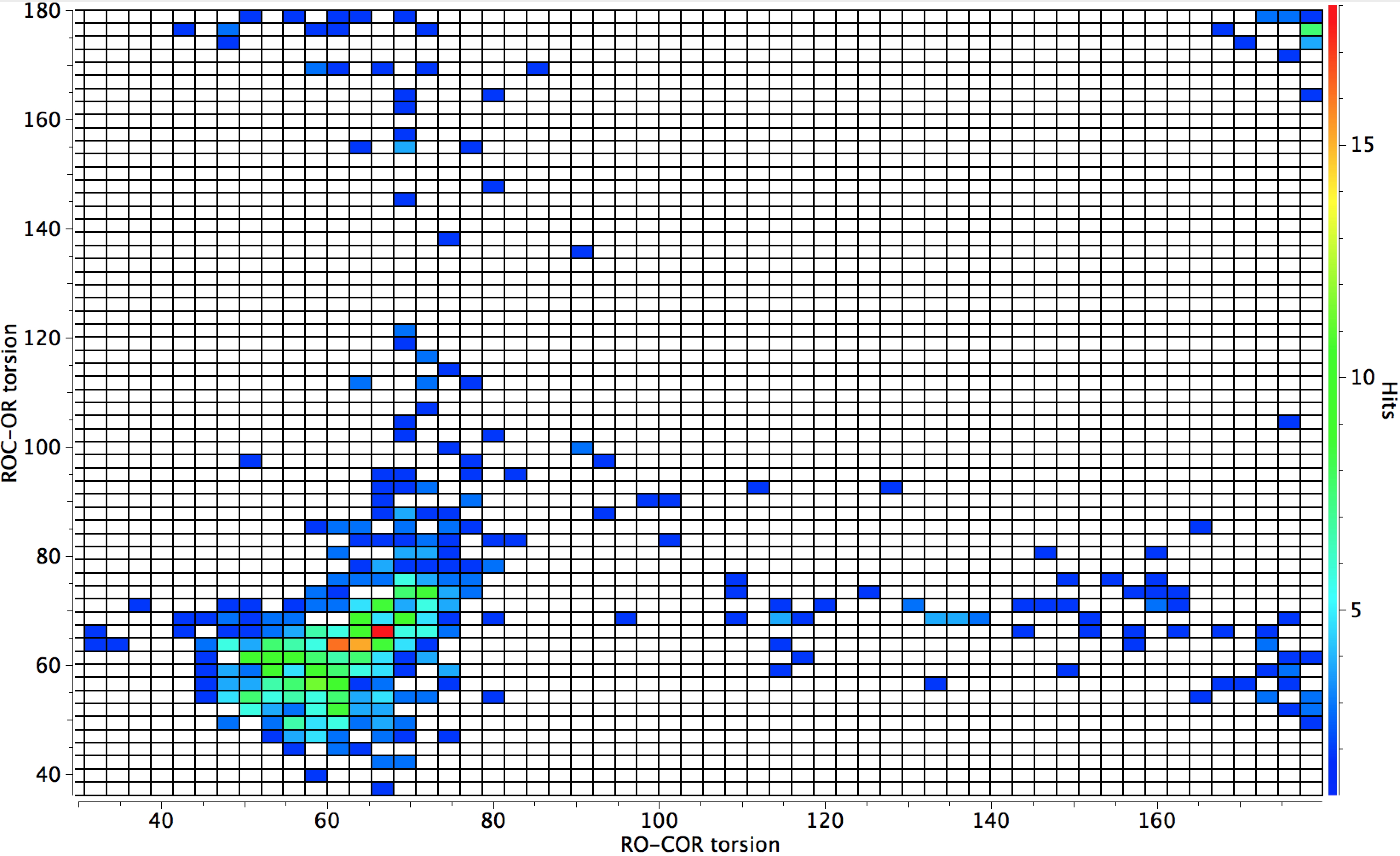
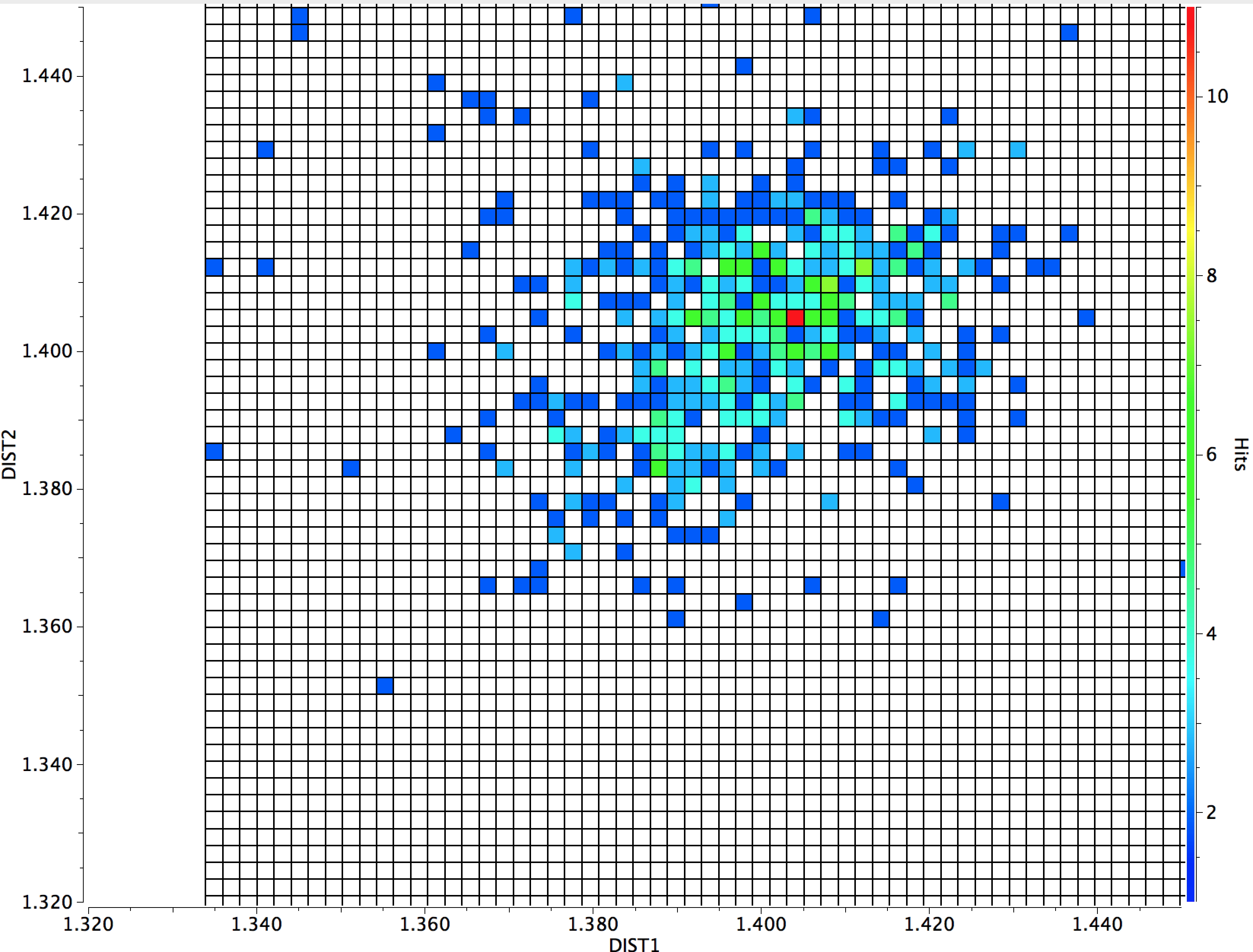
I also include a further constraint, that the diffraction data must be collected below 140K. The hotspot moves to ~ 55/60° indicating values free of some vibrational noise.
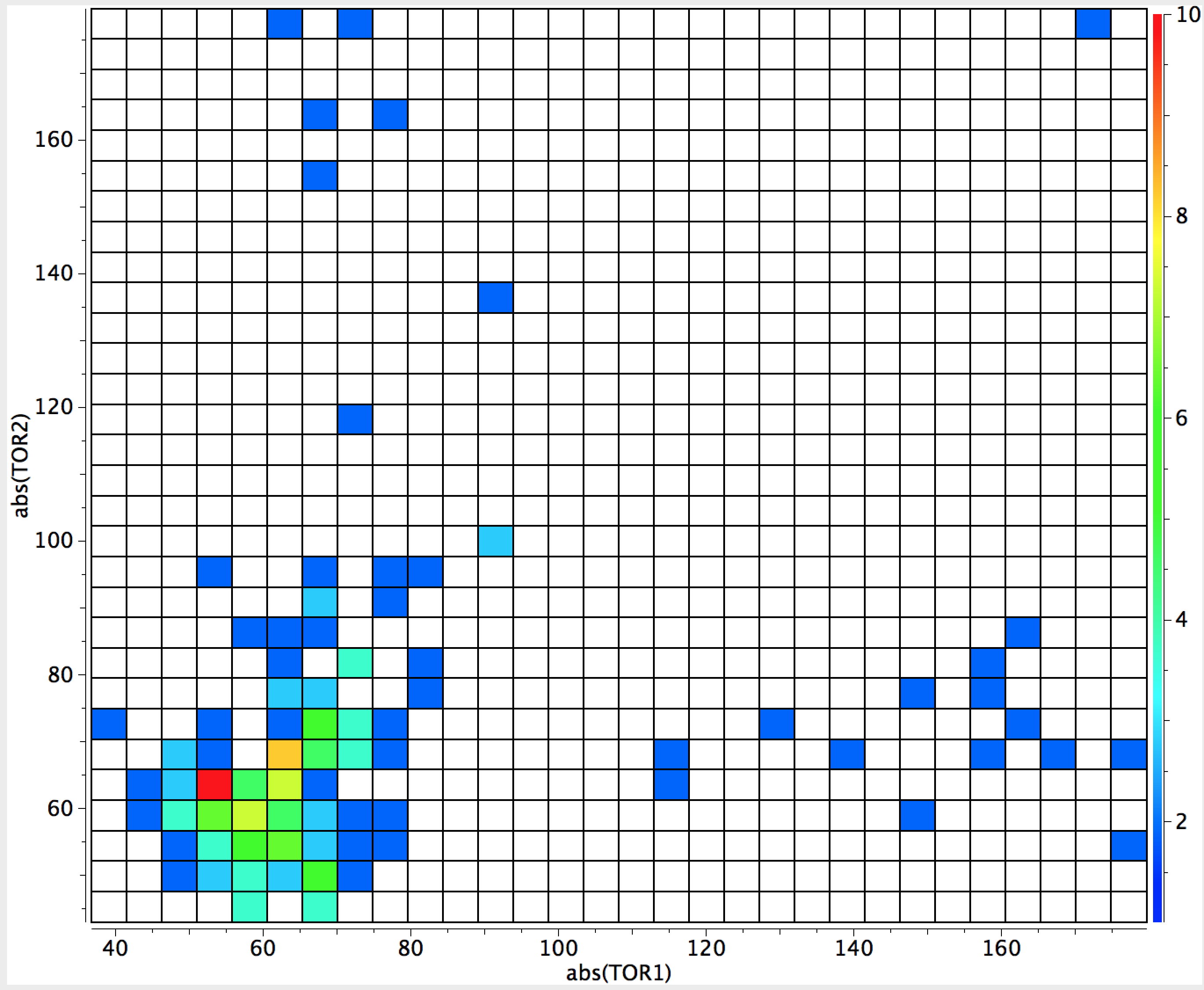
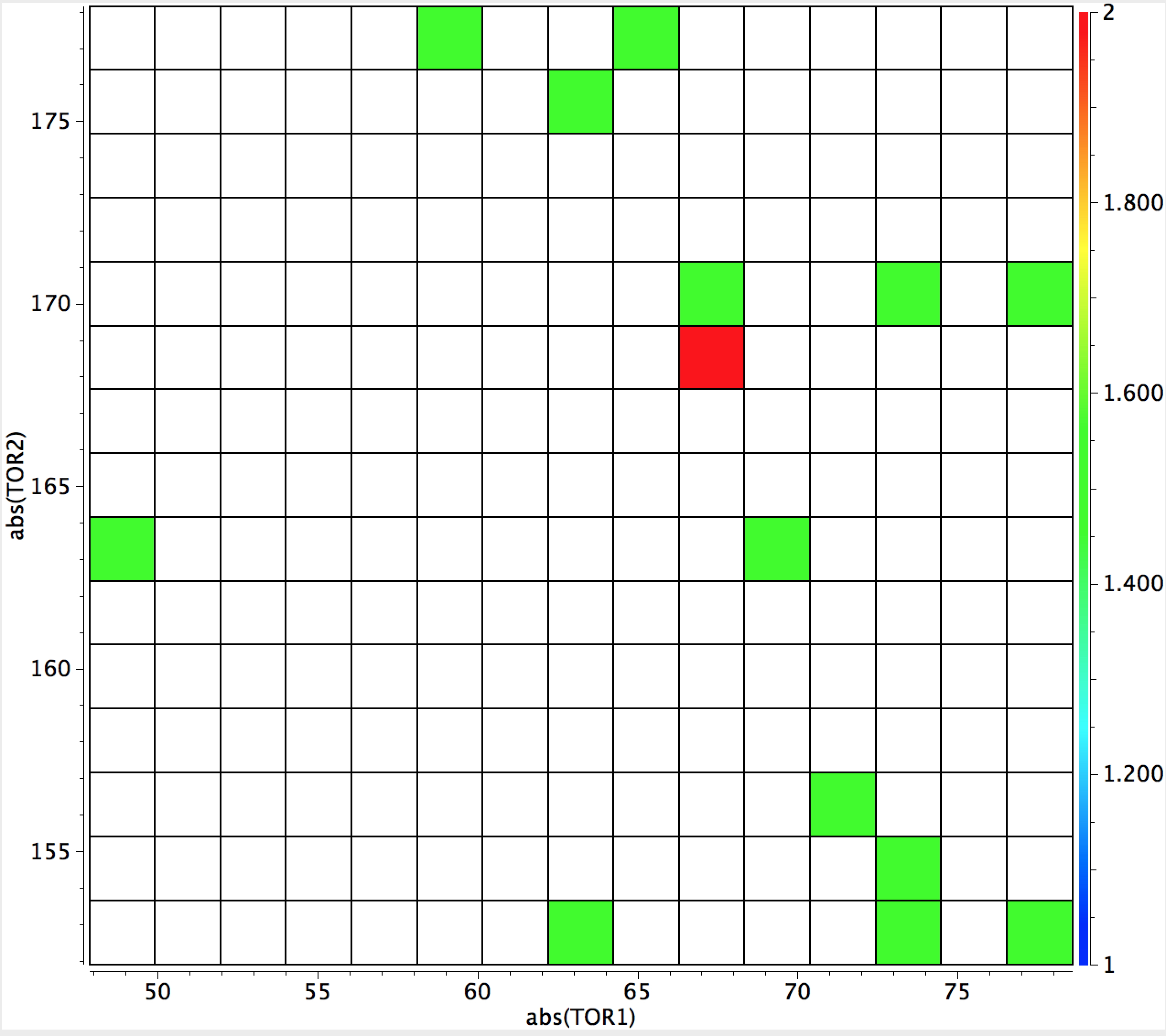
Interestingly, replacing oxygen with nitrogen reveals relatively few examples of the effect (C(NR2)4 is an exception). Replacing O by divalent S produces only 13 hits, with the surprising result (below) that in all of them only one S sets up an anomeric interaction. Arguably, the number of examples is too low to draw any firm conclusions from this observation.
‡Most diffractometers measure low angle scattering of X-rays by high density electrons. These are the core electrons associated with a nucleus rather than the valence electrons associated with lone pairs. Hence very few positions of valence lone pairs have ever been crystallographically measured.
Acknowledgments
This post has been cross-posted in PDF format at Authorea.
Tags: Alkane stereochemistry, Anomeric effect, Carbohydrate chemistry, Carbohydrates, Carbon–oxygen bond, Chemical bond, Ether, Lone pair, Physical organic chemistry, Quantum chemistry, Stereochemistry, Technology/Internet