A previous post posed the question; during the transformation of one molecule to another, what is the maximum number of electron pairs that can simultaneously move either to or from any one atom-pair bond as part of the reaction? A rather artificial example (atom-swapping between three nitrosonium cations) was used to illustrate the concept, in which three electron pairs would all move from a triple bond to a region not previously containing any electrons to form new triple bonds and destroy the old. Here is a slightly more realistic example of the phenomenon, illustrated by the (narcisistic) reaction below of a bis(sulfur trifluoride) carbene. Close relatives of this molecule are actually known, with either one SF3 of the units replaced by a CF3 group or a SF5 replacing the SF3 (DOI: 10.1021/ja00290a038 ).
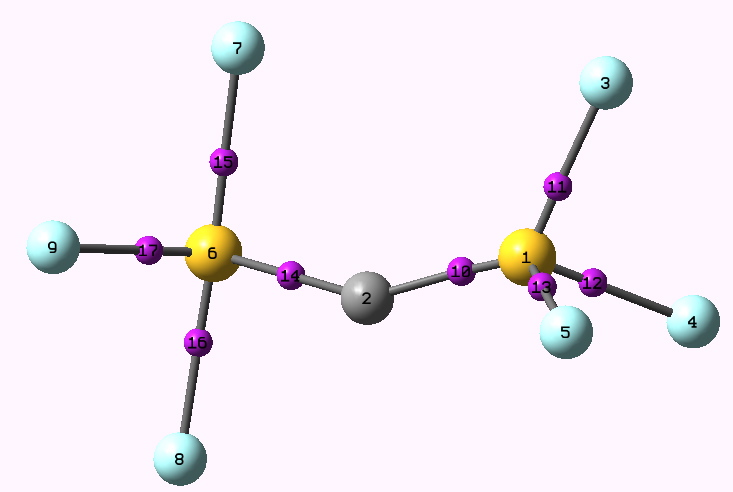
The two C-S bonds in this molecule are not the same (and similarly for the CF3 analogue), one being long (single), the other short (assumed triple), and the angle subtended at the central carbon is around 150° (B3LYP/cc-pVTZ calculation, DOI: 10042/to-3643). The transition state for interconverting one form to the other would presumably correspond to the concerted movement of two pairs of electrons from one CS region to the other as shown above, not so much a Ménage à trois, as a Ménage à deux! The transition state itself (DOI: 10042/to-3644) has C2 symmetry, with a calculated free energy barrier of 31 kcal/mol and ν 284i cm-1 for the bond shifting process.

Transition state for bond equalisation. Click for animation
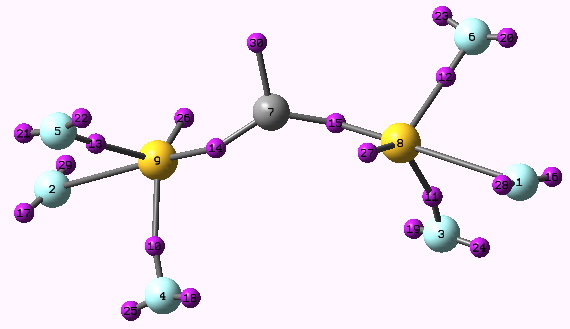
The molecule above does have a further point of interest; one of the sulfur atoms (the triply bonded one) is approximately tetrahedral in coordination, whilst the other has a “T-shape”. An inorganic chemist would describe one sulfur as tetravalent (oxidation state IV), the other as hexavalent (oxidation state VI) and the equilibrium between them a dismutation of the two oxidation states. Does this have any reality? The ELF method has been mentioned a number of times in these posts, and it is applied here to seek an answer. The ELF basin centroids are shown below.

ELF basins for F3SCSF3. Click for 3D
The integrations are as follows: 14 = 2.24 (a single C-S bond), 30=1.66 (an incipient carbene forming, as implied above), 13+15+16 = 4.34 (a reasonably persuasive triple bond, comprising, unusually, three separated basins). The fluorines 2, 3 and 6 all exhibit bonding basins to the S (respectively 2.17, 2.17 and 2.09), but fluorines 1,5 and 4 do not! Sulfur 8 additionally has a lone pair, 29=2.31, but sulfur 9 does not. One aspect of this analysis is the nature of the triple bond between S9-C7. Because the three basins are separate, does that mean that the bond cannot rotate about its axis?
An alternative AIM analysis is shown above. Now, the CS triple bond is reduced to a single bond critical point (BCP), labelled 10. AIM allows a property known as bond ellipticity to be computed at that BCP. Typically, single and triple bonds have ellipticities close to zero, whilst double bonds have a value of around 0.4 to 0.5. That for point 10 is 0.18, which seems to support the ELF analysis above. Pretty unsual bonding it would have to be agreed!
But what of the original question posed at the start in the diagram; do two pairs of electrons move away together from one triple bond to form another. A further ELF analysis at the transition state for this process reveals that in effect the two pairs do different things. One localizes onto the carbon, to form a proper carbene, the other becomes a sulfur lone pair. So the valence dismutation involves three pairs of electrons, not two as shown at the start, with each pair doing its own thing.
Tags: animation, calculated free energy barrier, Hypervalency, inorganic chemist, Interesting chemistry
[…] Henry Rzepa Chemistry with a twist « Ménage à deux: Non-classical SC bonds. […]
[…] transition state, it transpires is not on the pathway to dissociation, but is a double [1,2] migration (in this it is related to the coarctate mechanism, which involves two bonds […]
[…] † Another example was the topic of this post. […]