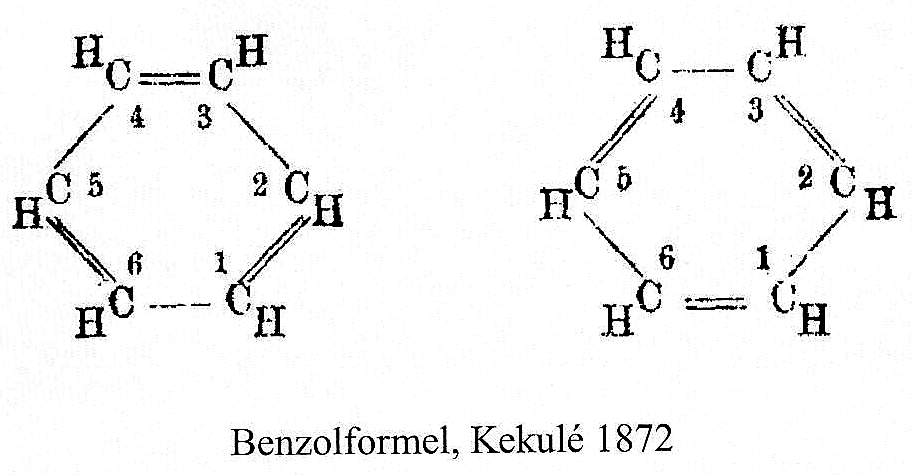
In the preceding post, a nice discussion broke out about Kekulé’s 1872 model for benzene.[1] This model has become known as the oscillation hypothesis between two extreme forms of benzene (below). The discussion centered around the semantics of the term oscillation compared to vibration (a synonym or not?) and the timescale implied by each word. The original article is in german, but more significantly, obtainable only with difficulty. Thus I cannot access[1] the article directly since my university does not have the appropriate “back-number” subscription.‡ So it was with delight that I tracked down an English translation in a journal that I could easily access.[2] Here I discuss what I found (on pages 614-615, the translation does not have its own DOI).
The translation is by no other than Henry Armstrong, whose own contributions I have documented elsewhere. The pertinent points (it’s a long explanation) seem to be:
- Kekulé does not use the word oscillation anywhere. This seems to have been added by subsequent commentators.
- He does describe the atoms as being in continuous movement, actually using the very modern term intramolecular motion (as translated of course).
- He also describes this motion as returning to a mean position of equilibrium, and the separate atoms as possessing rectilinear motion, striking and recoiling against adjacent partners.
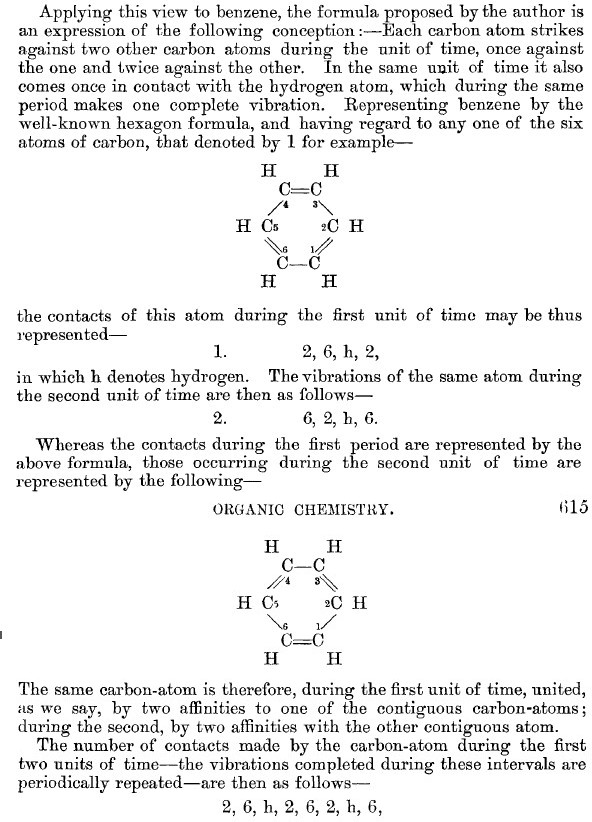
- He finally concludes by describing at some length what happens during two units of time involving what we would regard as one complete vibration to return the atoms to their starting point. This description is couched in words, and refers to what we would now call a normal (vibrational) mode evolving in time. You can see that written description below for yourself (in translation). It IS quite verbose; if ever a case could be made for replacing 1000 words with one picture, this is it!
Perhaps I can attempt to replace the (1000?) words above with that one picture (below). Here, I think Kekulé does manage to complicate things by including a hydrogen (h) as part of his scheme. Carbon C1 is described as contacting C2, and then immediately a hydrogen (although since he does not number the hydrogens it is not absolutely clear he means the hydrogen on carbon 2 at this stage). The modern equivalent below shows relatively little motion from the (light) hydrogen atoms, and certainly no obvious contact between e.g. C1 and any hydrogen other than the one it is bonded to.
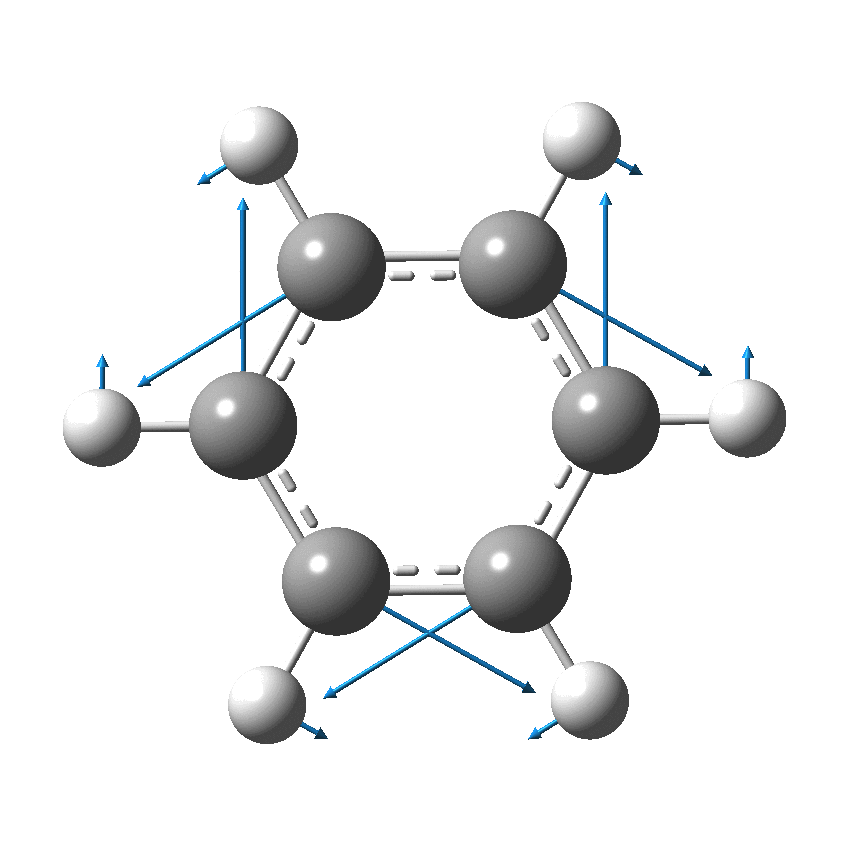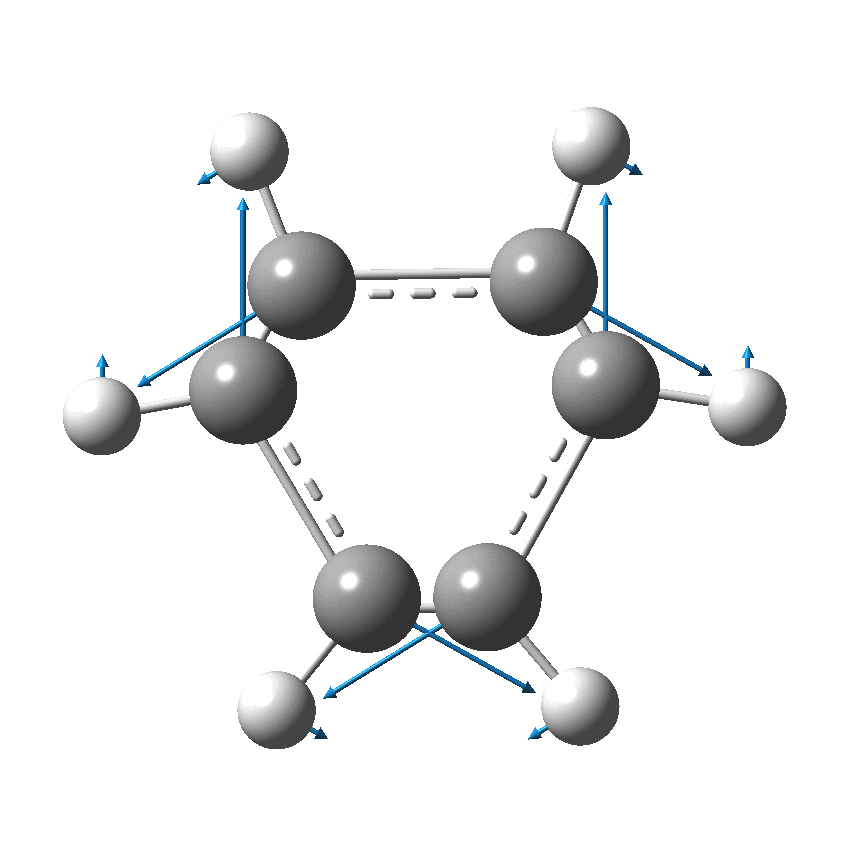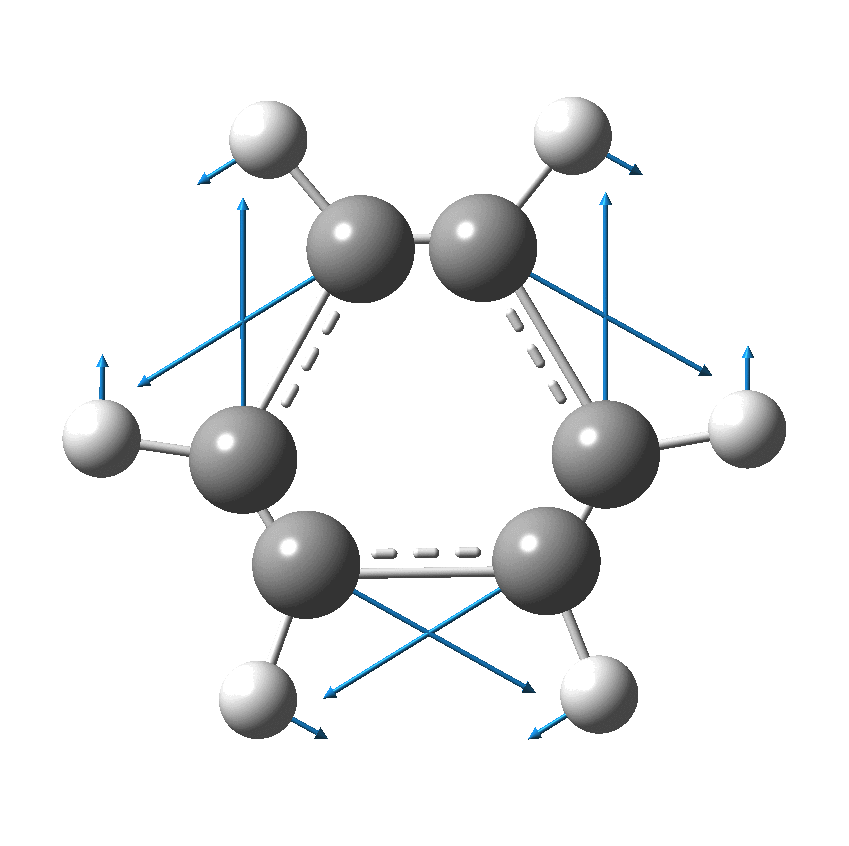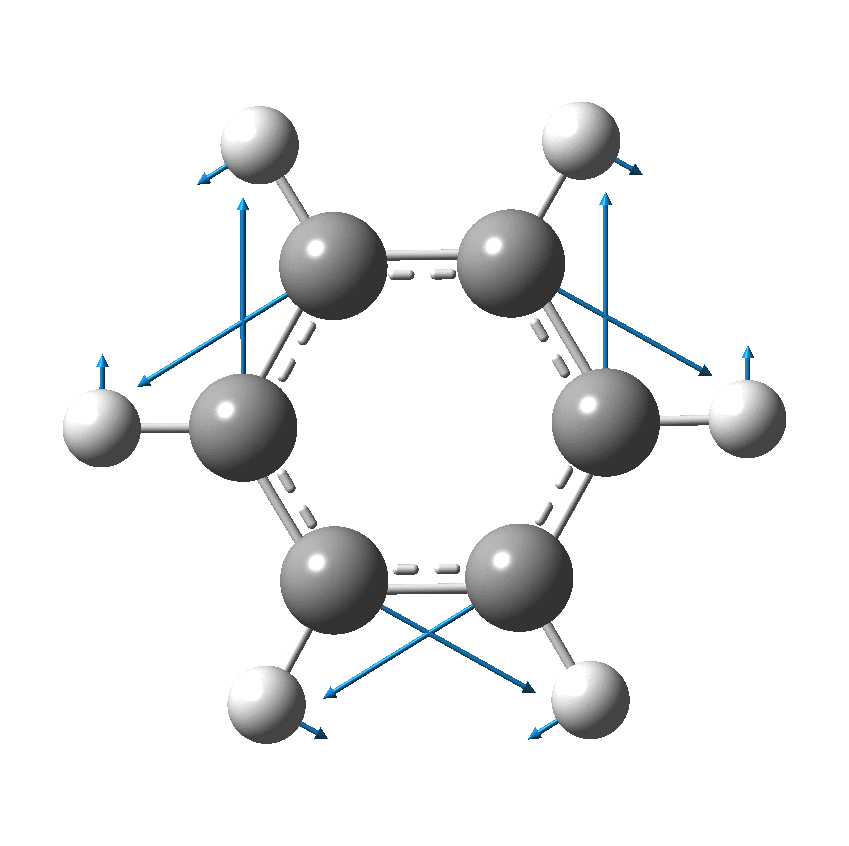
We now replace the description above by using far more concise vectors to describe the movement of the atoms with respect to time. And of course Kekulé had no real idea of how long his cycle took (only that it must be short as inferred from the laboratory observation of not being able to isolate geometric isomers, perhaps shorter than 100 seconds?); we now know that it is about 10-14 s. Commentators to this day describe this as Kekulé’s oscillation hypothesis, but since Kekulé did not use the term at all but did use (thrice) the word vibration we really should call it his vibration hypothesis, as indeed Paul Schleyer noted in his comment on the original post.
‡There is little doubt that historical researches have become severely endangered by the increasing lack of access to older issues of many journals. In some cases, older can mean as little as ten years!
References
- A. Kekulé, "Ueber einige Condensationsproducte des Aldehyds", Justus Liebigs Annalen der Chemie, vol. 162, pp. 77-124, 1872. https://doi.org/10.1002/jlac.18721620110
- "Organic chemistry", Journal of the Chemical Society, vol. 25, pp. 605, 1872. https://doi.org/10.1039/js8722500605
Dear Henry, I think that Kekule’ realized that benzene was fully symmetrical (D6h we would say now), but struggled to reconcile this conclusion with the D3h cyclohexatriene formulations required by the structural theory of the mid 19th century. His long-winded “vibration hypothesis” (“oscillation theory” NEVER should be attributed to him)!, was the best he could come up with (although vibration theory was poorly understood in his day and electronic structure theory was far in the future). To me, a vibration has a much shorter time scale than an “oscillation.” And I infer a double well potential from the latter, but only a single minimum from the former.
Since you confirm that Kekule’ never proposed “oscillation” or used the term, who introduced it into the literature and when? I hope authors and textbooks will discontinue repeating this error and showing two equilibrium arrows between two D3h benzenes.
Dear Henry, I think that Kekule’ realized that benzene was fully symmetrical (D6h we would say now), but struggled to reconcile this conclusion with the D3h cyclohexatriene formulations required by the structural theory of the mid 19th century. His long-winded “vibration hypothesis” (“oscillation theory” should NEVER be attributed to Kekule’!) was the best he could come up with (although vibration theory was poorly understood in his day and electronic structure theory was far in the future). To me, a vibration implies a much shorter time scale than an “oscillation.” And I infer a double well potential from the latter, but only a single minimum from the former.
Since you confirm that Kekule’ never proposed “oscillation” or used the term, who introduced it into the literature and when? I hope authors and textbooks will discontinue repeating this error as well as showing two equilibrium arrows between two D3h benzenes, and attributing this misconception to Kekule’.
Thanks Paul. We can agree that an oscillation is slower than a vibration (no nuclear motion could be faster than a vibration!).
When did oscillation first appear? Well, possibly in third volume of the text book Kekule published with Anschütz and Schultz which appeared 1882, and where Dieter Lenoir thinks that oscillation theory is mentioned. If anyone has access to this book, perhaps this could be checked?
Of course, the “history of the chemical arrow” is of itself an interesting topic. I attach here a more or less complete collection (using ChemDraw), where of course nowadays the equilibrium arrow and the resonance arrow are clearly distinguished. So at some stage the former stopped being used and the latter took over. I think surely all books nowadays (and perhaps the last 40 years) use the latter?
Just for completion I show here the original german version of Kekule’s article, which Lucas has kindly sent me.
Paul asks “who introduced oscillation into the literature and when?” Well, a few months after Kekule in 1872, this commentary by A. Michaelis appeared: doi: 10.1002/cber.187200501139 It shows a modern vector diagram for the vibration, and the description “oscillation”. My German is not good enough to give context to this use. Can anyone help?
Bravo, Henry, in locating Michaaelis’s use of the word “oscillation” in his 1872 paper on Kekule’s benzene model. But he also describes Kekule’s “complicated kind of vibrations {Schwingungen}.” My American dictionary defines “vibrate” and “oscillate” somewhat differently; I don’t know the distinctions of German usage.
I’m in Germany now, but haven’t gotten the whole paper yet nor consulted with
colleagues. I’ll report later.
I think the whole discussion is based on a flawed assumption that there is a “gradual” progress in science so Kekule’s idea of vibrations are somehow a prototype of our current understanding of the structure of benzene. This is “not the case. As Thomas Kuhn discussed in his “The structure of scientific revolutions” history of science is divided into “paradigms” and the concepts emerging in each paradigm is specific to that paradigm and usually “incommensurable” (not translatable easily in a new paradigm). Kekule’s view (as well as his contemporaries) was rooted in “chemical atom” paradigm that must be distinguished from our present day paradigm that is “physical atom” (for a good discussion see “The history of chemistry” by John Hudson). In the period of chemical atoms paradigm most chemists did not imagine atoms as real particles/systems and chemical structures were just “codes” to compress the chemical facts known for a compound (I mean various modes of chemical reactions). Thus the structural formulas were depicted as 2D objects and this remains the case until 1874 when Van’t Hoff and Le Bel proposed the 3D structural formulas imaging atoms as “real” entities (even then eminent chemists like Kolbe was quite skeptical of such ideas. For a good discussion see “The quite revolution” by Alan Rocke). So, any “modernization” of Kekule’s ideas must be down with caution (a good source on the history of evolution of ideas on benzene is a two part review paper by Stephen Brush: Stud. Hist. Phil. Sci. 30, (21-79, 263-302), 1999). Even more, in my point of view, Kekule’s idea on vibration of atoms is a good example of “ad hoc” modification of a theory that philosopher’s of science are usually unhappy with them. Kekule knew that his structural theory predicted 4 isomers for di-substituted benzene (two instead of one ortho isomers) so he invented (then quite speculative) the vibration theory to circumvent the plain discrepancy with then known experimental fact that just 3 isomers were known. His defense of this theory was quite shaky in his time and this remains to be case for more than half a century. This explains why people proposed a lot of “alternative” structural formulas for benzene without invoking any “dynamics” theory on atoms’ vibrations ; Kekule’s vibration theory was not satisfying his contemporaries. One could ask why just in the case of benzene Kekule used “dynamic” view whereas for other molecules just “static” representation of atoms was just sufficient? Where was the role of vibration in other molecules? Why benzene must be treated separately?
Our present day understanding of benzene’s chemical structure is based on Pauling’s “quantization” of Ingold’s mesomerism that he named resonance. This has nothing to do with Kekule’s vibrations and is a pure quantum mechanical effect affecting just the “electronic” structure. Thus, we don’t nowadays invoke any type of “vibration theory of atoms” to explain why benzene behaves differently from most other hydrocarbons.
In reply to shan’t shahbazian regarding “Kekule’s vibrations as a pure quantum mechanical effect”.
I am arguing that Kekule’s vibrations are very much alive and well, and really are vibrations of contemporary interest. Kekule’s normal mode vibration is at the core of our understanding of not just benzene but the whole host of higher order annulenes, up to say [26]-annulene or beyond. This vibration is a subtle blend of both the electronic and nuclear forces. To understand the structure of these annulenes means understanding Kekule’s vibration. At its core is whether the force constant defining the (harmonic) vibration is +ve (as it is for benzene) or -ve (as it would be for eg cyclobutadiene) and the origins of what determines that sign for the higher order systems. Is the sign dominated by the σ or the π manifolds? Equally interesting is the value of this force constant as perturbed by the detail of the structure, what increases it, what decreases it?
Because this is such a modern problem, it is even more important that we identify how our understanding of this very very subtle property evolved, from before Kekule and indeed after him. Kekule’s vibration has been at the core of our understanding of cyclic conjugated molecules for 140 years, and continues to be so. It is not just a historical curiosity, set in its time, and of no real relevance to modern chemistry.
Of course I am not arguing that normal mode analysis is a useful source of information for better understanding of bonding in benzene and annulenes (the recent works of Prof. Cremer witness its usefulness. See for instance Curr. Org. Chem. 14, 1524 (2010)). What I am insisting is that Kekule vibration theory must be understood in its historical context and has no relevance not in its approach and nor in its goal to present day normal mode analysis. Any resemblance (translation) to modern ideas in my opinion is superficial. Interestingly, Pauling also termed his canonical resonance forms as Kekule structures though plainly he had no nuclear vibration in his mind but just naming VB electronic structures at the equilibrium geometry. Seemingly, there is a tendency in chemistry to translate the old concepts into modern ones. If this is just naming then there is no problem however, if this is intentional seeking for “continuation” of he evolution of chemistry in the last three centuries then it is in my opinion misleading. In current semester I was teaching history of chemistry to freshmen and all I wanted to emphasize them is that chemistry had not been always like today! Not only its instrumentation but also its basic understanding of the nature of matter. Probably benzene is a good example since it was always in focus in the last 150 years.
I think we can have an interesting discussion on the point “Any resemblance (translation) to modern ideas in my opinion is superficial.”.
I do disagree that the resemblance is superficial. What Kekule describes, and what Michaelis clarifies, does not in my opinion, merely superficially resemble what we now call a normal vibrational mode. Michaelis’ displacement vectors do seem to strongly resemble the modern displacement vectors qualitatively at least. The resemblance I feel is not superficial.
I accept that the nucleus as a point particle had not yet been discovered, and that some people such as Faraday felt that atoms were merely the intersection points of lines of force in a molecular electromagnetic field. In such a field theory, a classical model of particles vibrating in a (quadratic) potential would not make sense perhaps. But lots of people did believe in point atoms, and so such models would have made complete sense to them.
What is equally of interest is a historical understanding of how any model morphs. Thus the mechanical model (based on Young’s modulus, ie a force constant) only really needs to morph when confronted with a new phenomenon such as eg tunnelling. The analogy to eg Newtonian physics morphing to Einsteinian physics springs to mind. The two theories do not simply superficially resemble each other. In the same manner, I do not believe we can say Kekule was wrong, but that he was only qualitatively accurate (for benzene).
I would also like to know whether Michaelis’ vector diagram for a vibration or an oscillation was original, or whether similar diagrams for other molecules were in fact well known by 1872. I have been told (but cannot verify at the moment because I cannot lay my hands on his booklet) that Loschmidt in 1861 had already also described motions of atoms about an equilibrium position that could perhaps be the precursor to molecular vibrations/oscillations?