Around 100 tons of the potent antimalarial artemisinin is produced annually; a remarkable quantity given its very unusual and fragile looking molecular structure (below). When I looked at this, I was immediately struck by a thought: surely this is a classic molecule for analyzing stereoelectronic effects (anomeric and gauche). Here this aspect is explored.
I start by listing the bonds around which interesting things might happen:
- C3-C4 has the gauche motif of a 1,2-diol
- Carbons 7 and 4 are anomeric centres, with the focus on bonds 1-7/7-6 and 6-4/4-5
- Bond 1-2 has the potential for a so-called α-effect, where the lone pairs on adjacent hetero-atoms are buttressed.
The crystal structure is shown below, annotated with pertinent bond lengths (trivial atom numbering). The dihedral 2-3-4-6 and 2-3-4-6 are respectively -51 and 72° (hence a double gauche at the 3-4 bond).

Click for 3D
First, an exploration of what might be happening around C4. The following is a search of the Cambridge crystal structure database, plotted for the two C-O bond lengths common to C4.
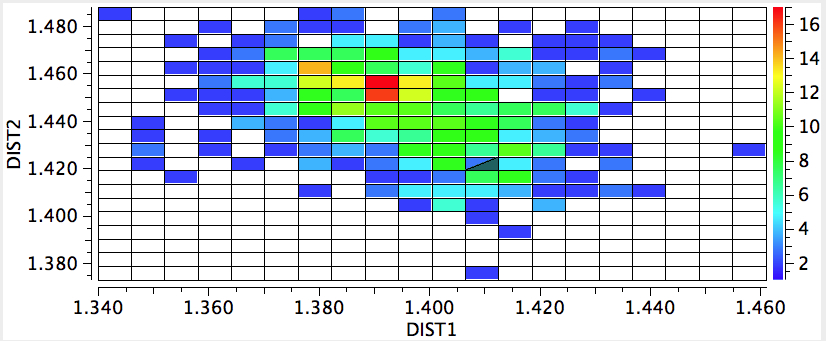 Here, DIST1 is C4-O6 and DIST2 is C4-O5. Notice the very pronounced asymmetry; at the red hotspot above, the most frequent occurrence is ~1.39 and 1.46Å respectively; artemisinin is more or less at that hotspot. This can be quantified by the NBO E(2) energies for the interaction of an oxygen lone pair antiperiplanar to the C-O σ* bond;
Here, DIST1 is C4-O6 and DIST2 is C4-O5. Notice the very pronounced asymmetry; at the red hotspot above, the most frequent occurrence is ~1.39 and 1.46Å respectively; artemisinin is more or less at that hotspot. This can be quantified by the NBO E(2) energies for the interaction of an oxygen lone pair antiperiplanar to the C-O σ* bond;
- Lp(O6)-σ*(C4-O5) = 21.2 kcal/mol which helps to account for the short C4-O6 and the long C4-O5 bonds.
- whereas the reverse donation of Lp(O5)-σ*(C4-O6) is merely 4.8 kcal/mol (normally the two donations are more or less equal, and hence so at the two C-O bond lengths).
- At the second anomeric centre of C7, Lp(O1)-σ*(C7-O6) = 19.9 kcal/mol
- whereas the reverse donation of Lp(O6)-σ*(C7-O1) is 5.7 kcal/mol, again highly asymmetric, as are the C-O bond lengths (1.413/1.441Å).
- Next, the gauche effect at C3-C4. The C4-H to C3-O2 donation is 6.4 kcal/mol, again contributing to the longer C-O length of 1.447Å.
Where such stereoelectronic interactions are asymmetric, one might expect enhanced reactivity. A good example of this are two stereoisomeric of a 7-ring herbicide[1] where one anomer with equal anomeric C-O lengths is a stable soil-persistent species, whereas the other with asymmetric lengths has a very short soil residency due to rapid hydrolysis. It might be tempting to speculate that some aspect of the activity of artemisinin may be due to such stereoelectronic asymmetries.
Finally, because it is virtually free to do so in a computational sense, I show the computed VCD spectrum[2] (covering the possibility that it is measured at some point). The calculated[3] optical rotation ([α]589 is +93° (obs ~+76°). Whilst the absolute configuration is not in any doubt, it is always nice to have further confirmations.
This post has DOI: 10.14469/hpc/12766
References
- P. Camilleri, D. Munro, K. Weaver, D.J. Williams, H.S. Rzepa, and A.M.Z. Slawin, "Isoxazolinyldioxepins. Part 1. Structure–reactivity studies of the hydrolysis of oxazolinyldioxepin derivatives", J. Chem. Soc., Perkin Trans. 2, pp. 1929-1933, 1989. https://doi.org/10.1039/p29890001929
- H.S. Rzepa, "Gaussian Job Archive for C15H22O5", 2014. https://doi.org/10.6084/m9.figshare.997360
- H.S. Rzepa, "Gaussian Job Archive for C15H22O5", 2014. https://doi.org/10.6084/m9.figshare.997463
Tags: C7 Lp, Cambridge, stereo-electronics