According to Herges[1],[2] the mechanism of single-step (concerted) reactions can be divided into three basic types; linear (e.g. substitution, elimination etc), pericyclic (e.g. Diels Alder) and a third much rarer, and hence very often overlooked type that was named coarctate. This is based on the topology of bond redistribution patterns, an explicit real example[3] illustrating:
It happens that this reaction bears a close similarity to epoxidation using peracid, the characteristic feature being that the central (spiro) atom has two bonds forming to it and two bonds breaking from it in both reactions.†,‡ I had noted for the latter reaction that in fact the bond redistribution, although concerted, was asynchronous. This asynchrony was represented by the green arrows preceding the blue ones (or vice-versa for the reverse reaction).
So here I decided to investigate if the same might be true of the coarctate reaction shown above (ωB97XD/6-311G(d,p)/SCRF=water.[4]
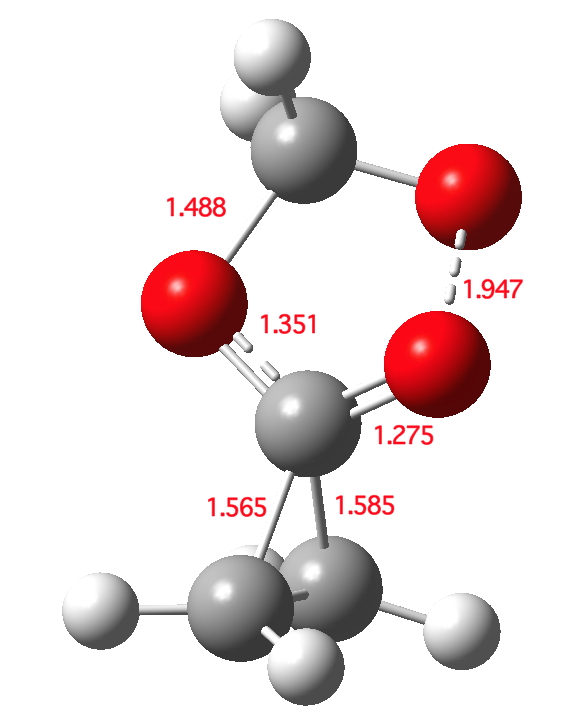
Click for 3D
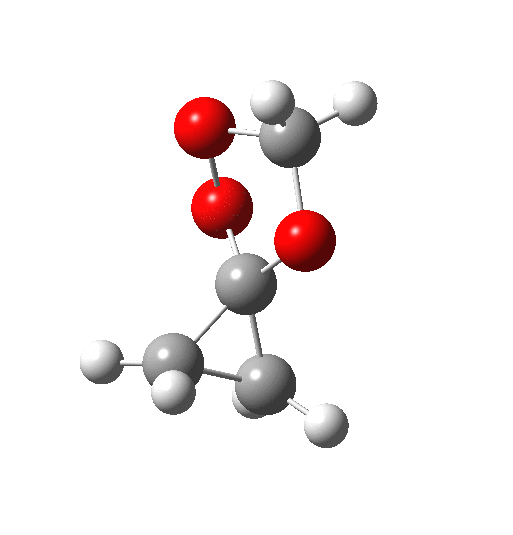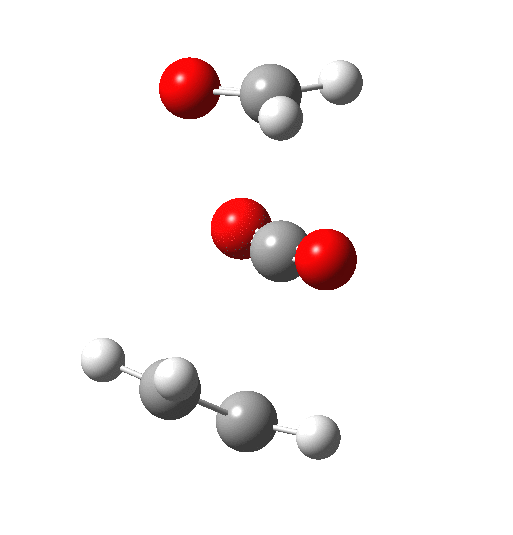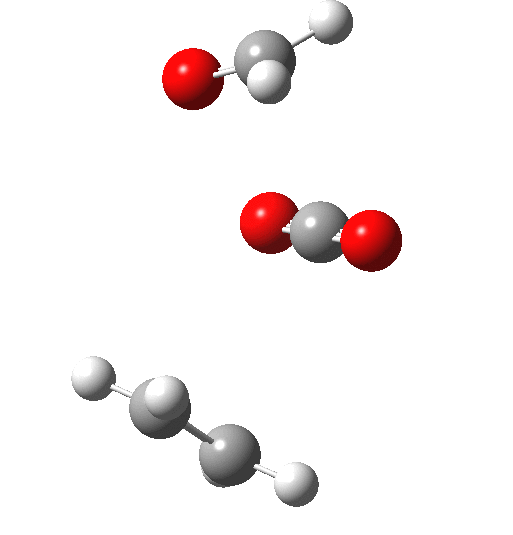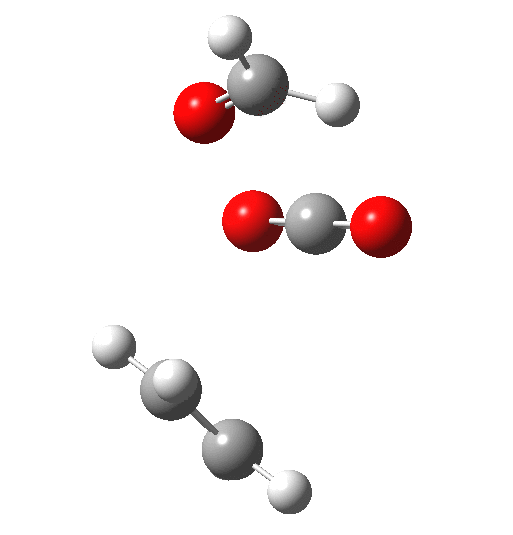
The transition state is indeed interestingly asynchronous. The O-O bond (shown green above) is clearly the first to break; neither of the C-C bonds has really started to do so at the transition state. But the process remains resolutely concerted.
The IRC above shows clearly that the reaction has a room-temperature barrier (i.e. it is a very facile process). But missing really from this process is any hidden intermediate either (there is the merest hint at IRC = -2). So this reaction is interesting for
- its classification apart from the normal two types of organic mechanism, as a coarctate type
- Its asynchrony in the bond redistributions
- but this asynchrony not resulting in any hidden intermediates.
† Another example was the topic of this post.
‡ One can contrive an even higher-order reaction (thus far un-named) in which (formally) three bonds break and three bonds form at a single atom.
References
- R. Herges, "Coarctate transition states: the discovery of a reaction principle", Journal of Chemical Information and Computer Sciences, vol. 34, pp. 91-102, 1994. https://doi.org/10.1021/ci00017a011
- B.S. Young, R. Herges, and M.M. Haley, "Coarctate cyclization reactions: a primer", Chemical Communications, vol. 48, pp. 9441, 2012. https://doi.org/10.1039/c2cc34026g
- C. Berger, C. Bresler, U. Dilger, D. Geuenich, R. Herges, H. Röttele, and G. Schröder, "A Spontaneous Fragmentation: From the Criegee Zwitterion to Coarctate Möbius Aromaticity", Angewandte Chemie International Edition, vol. 37, pp. 1850-1853, 1998. https://doi.org/10.1002/(sici)1521-3773(19980803)37:13/14<1850::aid-anie1850>3.0.co;2-b
- H.S. Rzepa, "Gaussian Job Archive for C4H6O3", 2013. https://doi.org/10.6084/m9.figshare.787693
Tags: Ozonolysis
Coarctate reactions is really a new concept. I’m still having hard time to understand it 🙁
[…] Chemistry with a twist « Coarctate reactions as a third fundamental organic-mechanistic type. […]