The butterfly effect summarises how a small change to a system may result in very large and often unpredictable (chaotic) consequences. If the system is merely on the edge of chaos, the consequences are predictable, but nevertheless finely poised between e.g. two possible outcomes. Here I ask how a molecule might manifest such behaviour.
Two examples of the molecule above are known, differing only in the nature of the R group.
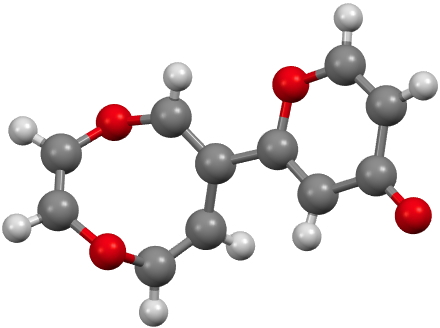 CUWWEW. Click for 3D. |
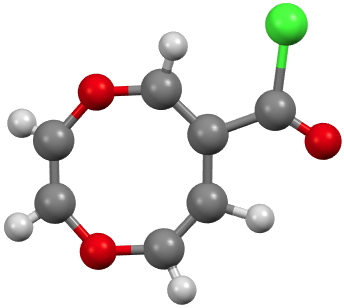 CUWWIA. Click for 3D. |
CUWWEW is strongly buckled and shows no sign of cyclic conjugation, with the double bonds localised. On the other hand the very similar CUWWIA is essentially planar, being so because it contains ten π-electrons in a planar ring and so is 4n+2 aromatic (n=2) and becomes delocalised. However, neither form is entirely happy; CUWWEW relieves ring string (in a flat 8-ring the internal angles are ~140°, a significant departure from that preferred for sp2 hydridisation) but looses any stabilisation from aromaticity. For CUWWIA the reverse is true.
Clearly these two effects are finely balanced for this system and the result is a pair of molecules on the edge of chaos, where a small change to the R group can tip the molecule over from one state to another.[1],[2] I may return to this particular theme in future posts, whereby two molecules which differ perhaps only in substituents, nevertheless adopt quite different geometries and properties.
References
- H.S. Rzepa, and N. Sanderson, "Aromaticity on the edge of chaos: An ab initio study of the bimodal balance between aromatic and non-aromatic structures for 10π-dihetero[8]annulenes", Phys. Chem. Chem. Phys., vol. 6, pp. 310-313, 2004. https://doi.org/10.1039/b312724a
- H.S. Rzepa *, and N. Sanderson, "Aromaticity on the edge of chaos: an<i>Ab initio</i>CCSD(T) study of the bimodal balance between aromatic and non-aromatic structures for 10-π-diheterocins and heteronins", Molecular Physics, vol. 103, pp. 401-405, 2005. https://doi.org/10.1080/00268970512331317796
Your post http://www.ch.imperial.ac.uk/rzepa/blog/?p=7678 gives other example of the molecules which differ perhaps only in substituents, nevertheless adopt quite different geometries and properties (aromatic vs. nonaromatic semibullvalene).
And there are many examples in carbocations (nonclassical norbornyl vs. almost classical 2-phenylnorbornyl etc.)
Yes indeed, there are probably many examples of such bipolar molecules. I am not sure if any classification or comprehensive collection exists in a review anywhere?
In terms of the carbocations, I think these perhaps are more of a continuum, rather than two discrete states. Also, for the 10π-systems, the molecules can exist in BOTH states, with a barrier separating them (as we showed using CCSD(T) calculations). I am not sure any examples of this type of behaviour have ever been identified for carbocations?
Another example that deserves attention is the class of molecule termed bond stretch isomers where the essential difference is one isomer with a long bond (normally eg C=S) and another with a short one. I am uncertain if these examples proved to be artefacts or not. Does anyone know? Certainly in this case, it is difficult to see what the origins of a barrier separating them might be.
One final example might be molecules which differ in their spin state. Thus many Fe complexes can exist in eg triplet or quintet depending on substituents.
[…] Chemistry with a twist « The butterfly effect in chemistry: aromaticity on the edge of chaos. […]
[…] Promoting the latter would produce a 10π-aromatic ring (for another example of such, see here). Indeed so![2] This 10π-system has five occupied π-MOs, a low energy delocalised σ-MO of […]